Home /
Expert Answers /
Chemistry /
titration-of-a-weak-diprotic-acid-with-strong-base-naoh-titration-of-a-diprotic-weak-acid-14-12-10-pa569
(Solved): Titration of a Weak Diprotic Acid with Strong Base NaOH Titration of a Diprotic Weak Acid 14 12 10 ...
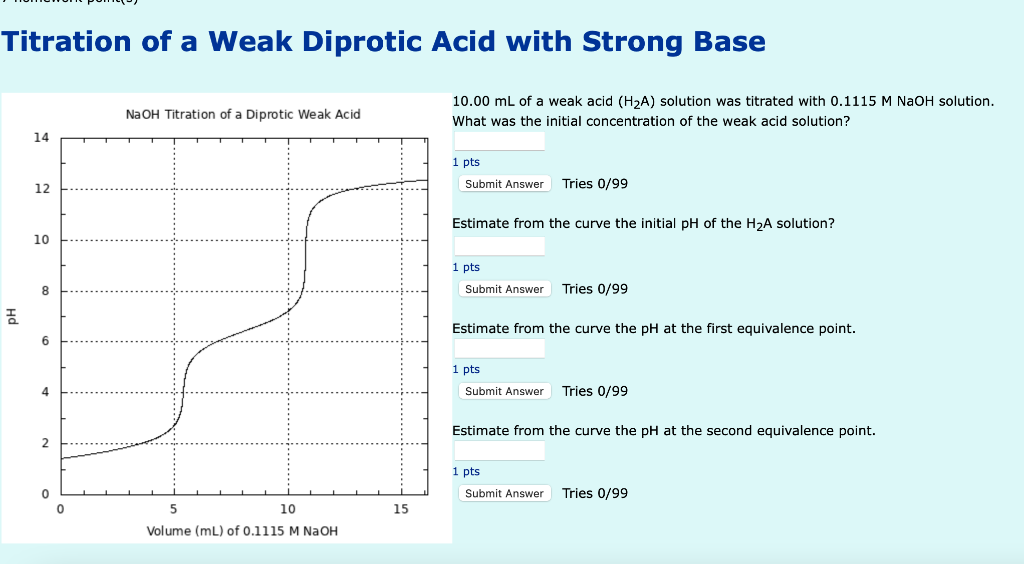
Titration of a Weak Diprotic Acid with Strong Base NaOH Titration of a Diprotic Weak Acid 14 12 10 8 6 4 2 0 E 0 5 10 Volume (mL) of 0.1115 M NaOH 15 10.00 mL of a weak acid (H?A) solution was titrated with 0.1115 M NaOH solution. What was the initial concentration of the weak acid solution? 1 pts Submit Answer Tries 0/99 Estimate from the curve the initial pH of the H?A solution? 1 pts Submit Answer Tries 0/99 Estimate from the curve the pH at the first equivalence point. 1 pts Submit Answer Tries 0/99 Estimate from the curve the pH at the second equivalence point. 1 pts Submit Answer Tries 0/99