Home /
Expert Answers /
Chemistry /
three-liquid-samples-of-known-masses-are-heated-to-their-boiling-points-with-the-use-of-a-heater-rat-pa701
(Solved): Three liquid samples of known masses are heated to their boiling points with the use of a heater rat ...
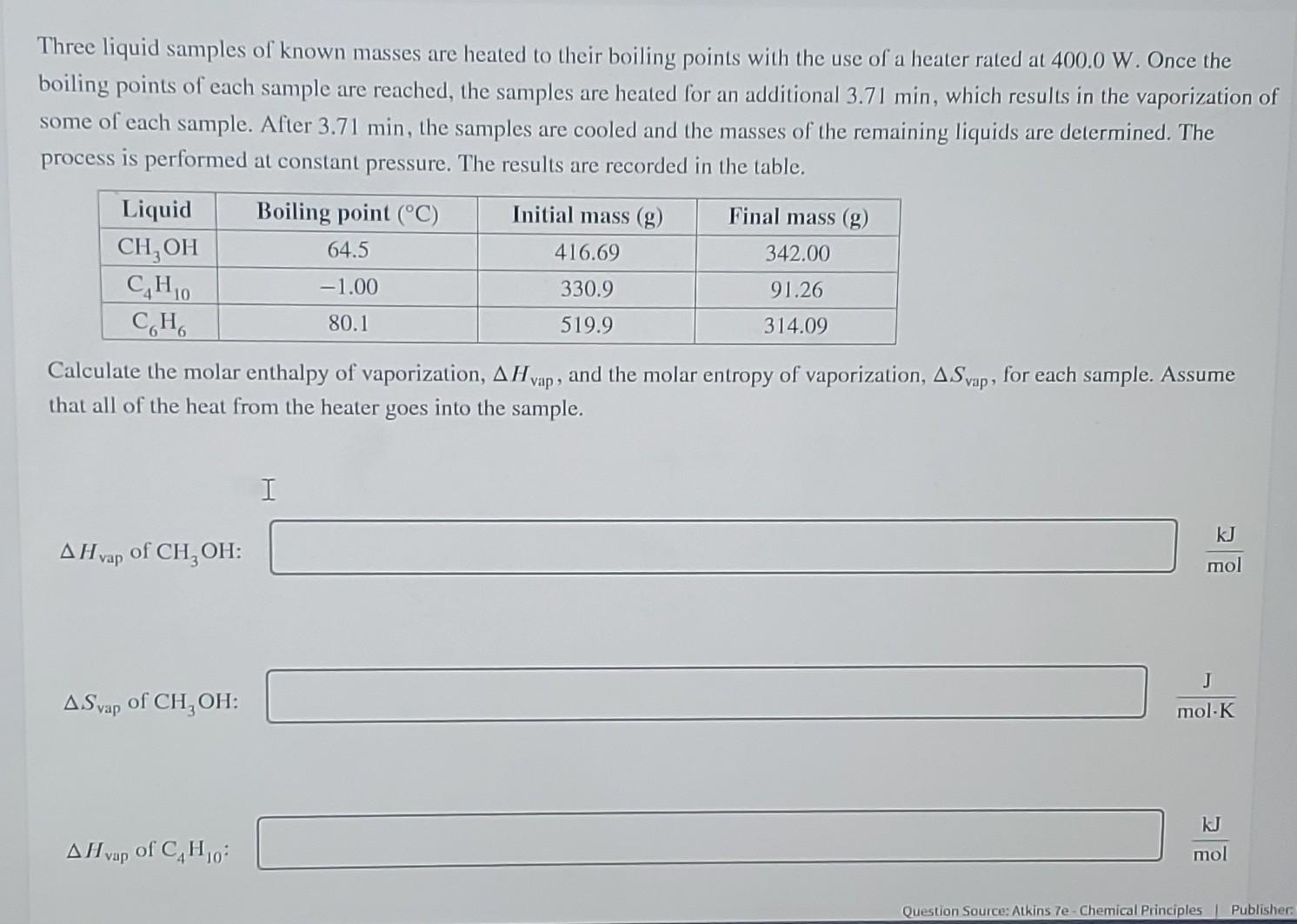
Three liquid samples of known masses are heated to their boiling points with the use of a heater rated at 525.0 W . Once the boiling points of each sample are reached, the samples are heated for an additional 6.10 min , which results in the vaporization of some of each sample. After 6.10 min , the samples are cooled and the masses of the remaining liquids are determined. The process is performed at constant pressure. The results are recorded in the table.
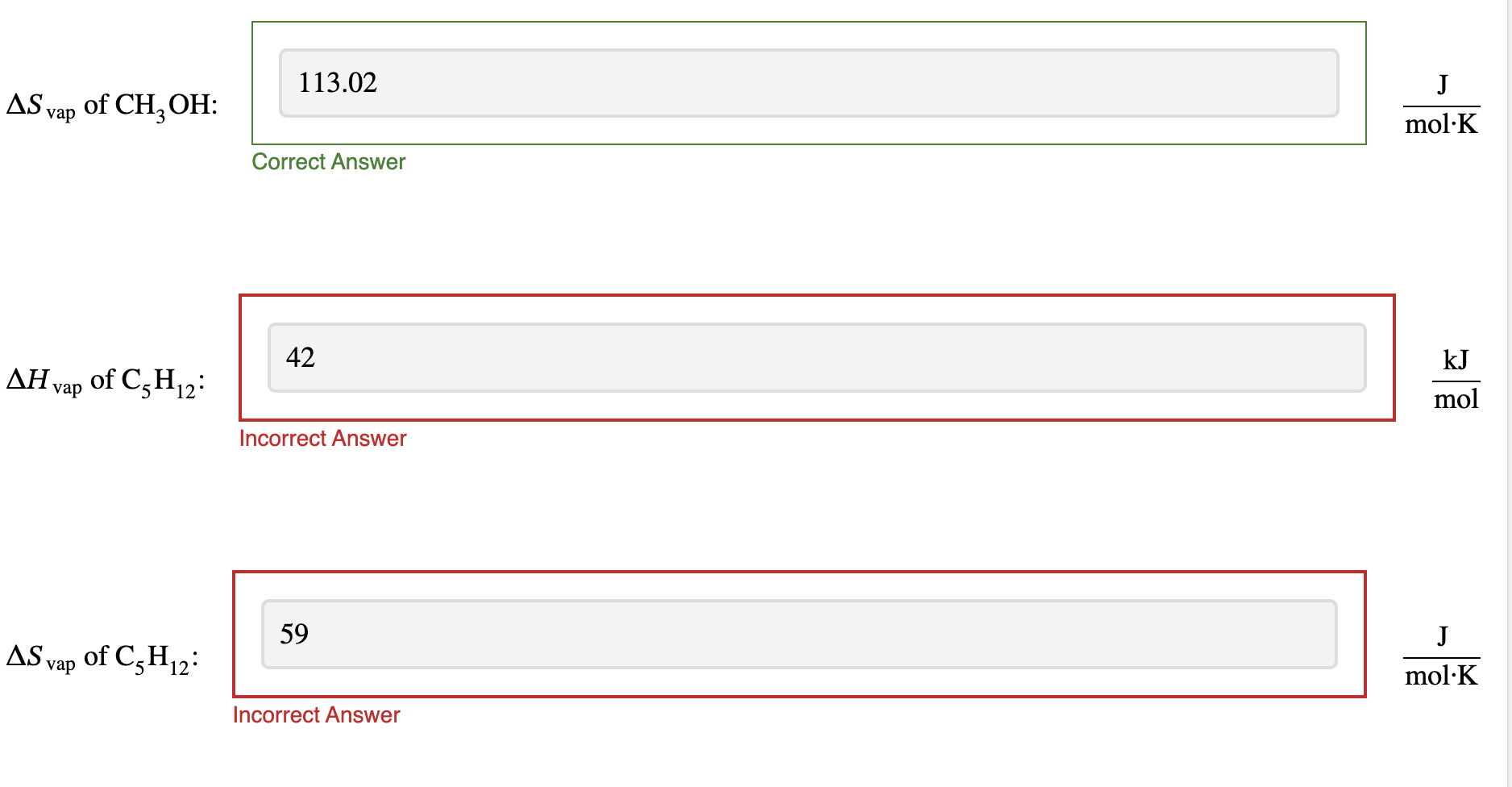
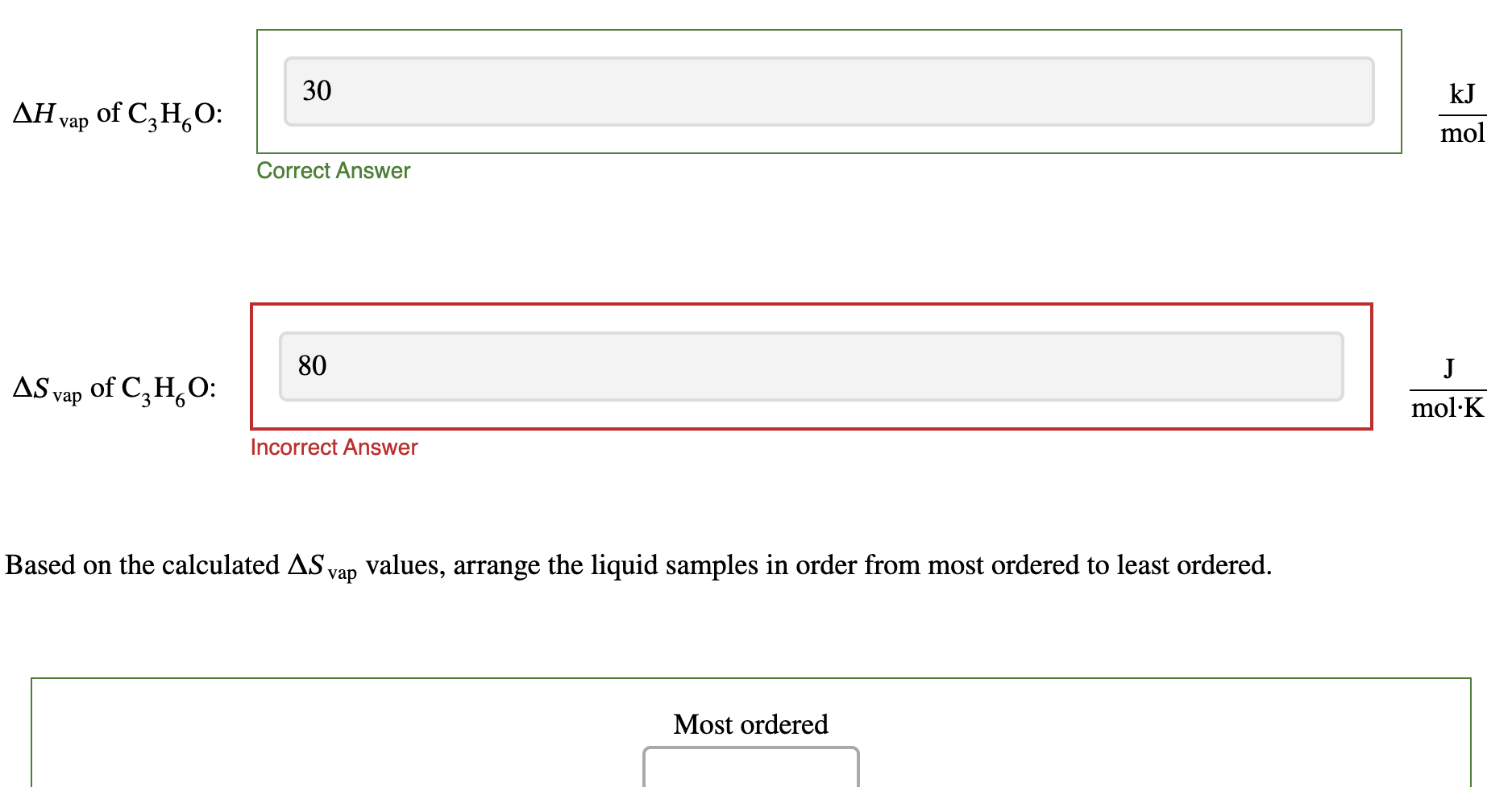
Calculate the molar enthalpy of vaporization \Delta Hvap , and the molar entropy of vaporization \Delta S_(vap) for each sample. Assume that all of the heat from the heatear goes into the sample.