Home /
Expert Answers /
Chemistry /
this-is-the-graph-i-calculation-of-the-enthalpy-of-disproportionation-reaction-of-h-pa155
(Solved): This is the graph: (i) Calculation of the enthalpy of disproportionation reaction of H ...
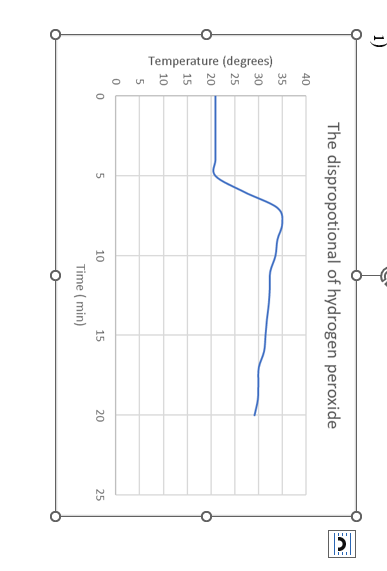
This is the graph:
(i) Calculation of the enthalpy of disproportionation reaction of H2O2 •
Determine the mass of the solution inside the flask.
From the graph you plotted of temperature versus time for the disproportionation of hydrogen peroxide determine the temperature change between the initial and final temperatures.
Experiment 1 Part 1B: Determining the Enthalpy of Reaction for the Disproportionation of Hydrogen Peroxide. 1. Add of the solution to the styrofoam cup using a graduated cylinder. Place a magnetic stir bar in the cup. Turn on the stirrer. 2. Examine the thermometer. You should read it to its full precision. 3. Add of to a graduated cylinder. This solution will be added to the solution at the 5-minute mark. 4. Measure the initial temperature of the solution. This is the zero-minute reading. Measure the temperature again after , and 4 , minutes. Remember to record your observations of the colour of the solution and the colour of the catalyst. 5. At the 5-minute mark, add the catalyst but do not measure the temperature. Record the color of the reaction mixture immediately after adding
the catalyst. 6. At the 6-minute mark, measure and record the temperature. Continue measuring and recording the temperature until the 20 -minute mark is reached. Remember to record your observation of the colour of the reaction mixture at the end of the experiment. 7. The reaction mixture just contains a dilute solution. 8. Draw a graph of temperature versus time.
1) The dispropotional of hydrogen peroxide