Home /
Expert Answers /
Chemistry /
this-is-the-experiment-for-the-synthesis-of-acetanilide-i-just-need-the-first-bullet-point-the-yie-pa939
(Solved): This is the experiment for the synthesis of acetanilide. I just need the first bullet point (the yie ...
This is the experiment for the synthesis of acetanilide. I just need the first bullet point (the yields) please
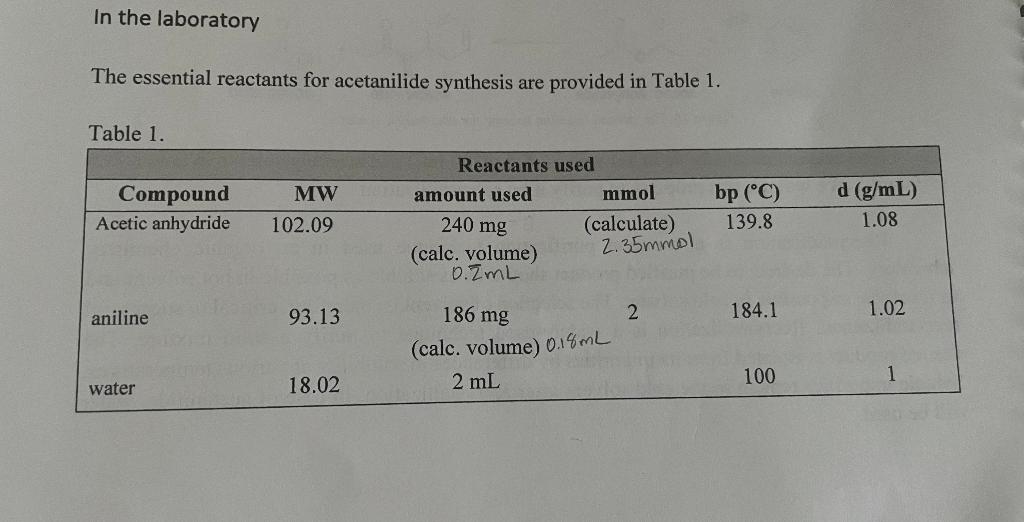
In the laboratory The essential reactants for acetanilide synthesis are provided in Table 1. Table 1. Compound Acetic anhydride aniline water MW 102.09 93.13 18.02 Reactants used amount used 240 mg (calc. volume) 0.ZML mmol (calculate) 2.35mmol 186 mg (calc. volume) 0.1% ml 2 mL 2 bp (°C) 139.8 184.1 100 d (g/mL) 1.08 1.02
• Report the theoretical yield (mass), obtained yield (mass and percent yield) and recovery (recrystallization) yields. Show your calculations in your notebook. ? Report NMR spectra analysis of your product and starting materials (report your data in a usual table format having complete peak analysis), draw structure of your compound on all your spectra. Discuss similarities/differences between product and reactants. Attach the original spectra at the end of your report. Provide m.p. analysis and literature value comparison. Cite your literature source. Report TLC analysis of aniline, commercial acetanilide and your recrystallized product. Report Rf values and discuss the results. Discuss all your data (yields, synthetic problems, NMR and TLC analysis); compare your data it with literature values. Comment on the time when the acetanilide was formed and the physical appearance of the product. In your discussion consider the following concepts: substitution, functional groups, reaction yield, recrystallization, polarity (TLC analysis). Summarize the main features of the report, the objectives, findings and conclusions. Briefly explain any possible sources of error relevant to your results. Have you successfully performed t? Was your synthesis/purification/analysis successful (yield...)?
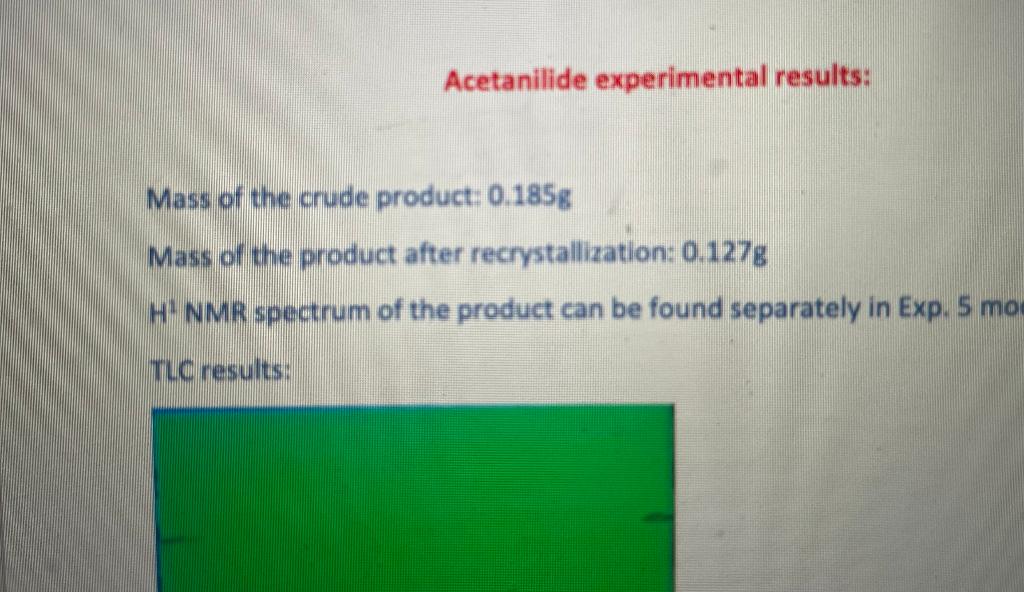
Acetanilide experimental results: Mass of the crude product: 0.185g Mass of the product after recrystallization: 0.127g H¹ NMR spectrum of the product can be found separately in Exp. 5 mo TLC results:
Expert Answer
The reaction scheme ; Reagents Molecular wt. Amount Density mmols equivalance Aniline 93.13 186 mg 1.02 2 1 Acetic anhydride 102.1 0.236 g /0.2 mL 1.08 2.3 1.16 Product, acetanilide 135.16