Home /
Expert Answers /
Chemistry /
thermodynamics-thank-you-q1-thermodynamics-and-thermochemistry-0-points-the-haber-bosch-process-is-pa239
(Solved): Thermodynamics thank you!! Q1 Thermodynamics and Thermochemistry 0 Points The Haber-Bosch process is ...
Thermodynamics thank you!!
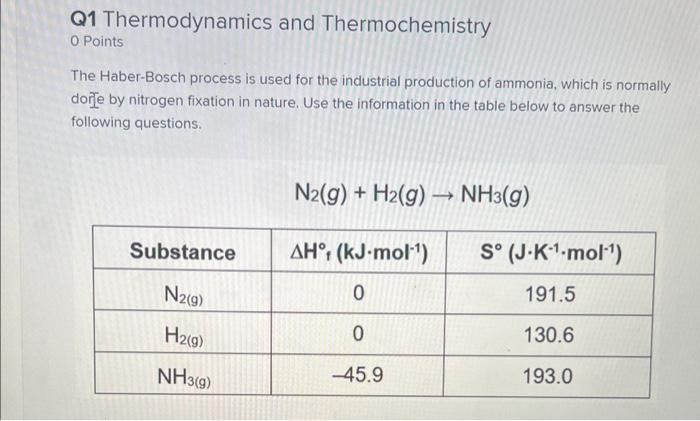


Q1 Thermodynamics and Thermochemistry 0 Points The Haber-Bosch process is used for the industrial production of ammonia, which is normally dorje by nitrogen fixation in nature, Use the information in the table below to answer the following questions. \[ \mathrm{N}_{2}(g)+\mathrm{H}_{2}(g) \rightarrow \mathrm{NH}_{3}(g) \]
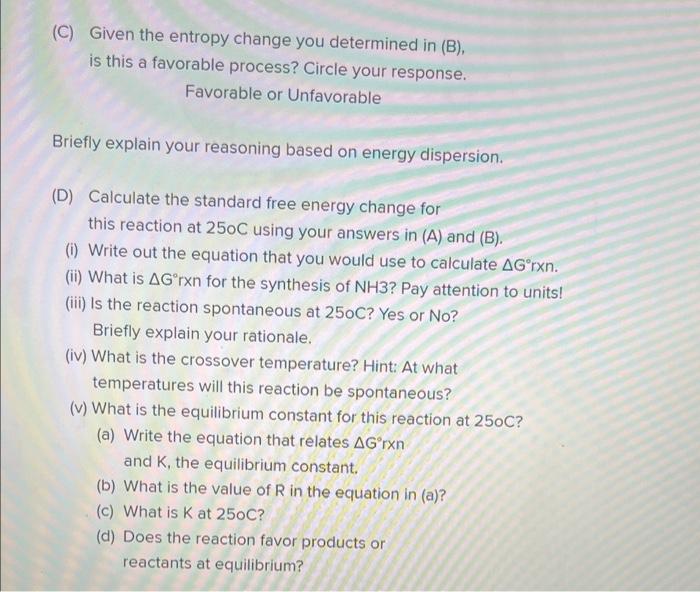
(C) Given the entropy change you determined in (B), is this a favorable process? Circle your response. Favorable or Unfavorable Briefly explain your reasoning based on energy dispersion. (D) Calculate the standard free energy change for this reaction at \( 250 \mathrm{C} \) using your answers in (A) and (B). (i) Write out the equation that you would use to calculate \( \Delta G^{\circ} \) rxn. (ii) What is \( \Delta G^{\circ} r x n \) for the synthesis of \( \mathrm{NH} 3 \) ? Pay attention to units! (iii) Is the reaction spontaneous at \( 250 \mathrm{C} \) ? Yes or No? Briefly explain your rationale. (iv) What is the crossover temperature? Hint: At what temperatures will this reaction be spontaneous? (v) What is the equilibrium constant for this reaction at \( 25 \mathrm{oC} \) ? (a) Write the equation that relates \( \Delta G^{\circ} \) rxn and \( K \), the equilibrium constant. (b) What is the value of \( R \) in the equation in (a)? (c) What is \( \mathrm{K} \) at \( 25 \mathrm{oC} \) ? (d) Does the reaction favor products or reactants at equilibrium?
(E) Use the following standard heats of formation to calculate \( \Delta H^{\prime} r x n \) for the reaction below. \[ \begin{array}{ll} & \mathrm{NH}_{3}(\mathrm{~g})+\mathrm{HCl}(\mathrm{g}) \rightarrow \mathrm{NH}_{4} \mathrm{Cl}(\mathrm{s}) \\ 1 / 2 \mathrm{~N}_{2}(\mathrm{~g})+11 / 2 \mathrm{H}_{2}(\mathrm{~g}) \rightarrow \mathrm{NH}_{3}(\mathrm{~g}) & \Delta \mathrm{H}^{\circ} \mathrm{f}=-46.1 \mathrm{~kJ} / \mathrm{mol} \\ 1 / 2 \mathrm{H}_{2}(\mathrm{~g})+1 / 2 \mathrm{Cl}_{2}(\mathrm{~g}) \rightarrow \mathrm{HCl}(\mathrm{g}) & \Delta \mathrm{H}^{\circ} \mathrm{i}=-92.3 \mathrm{~kJ} / \mathrm{mol} \\ 1 / 2 \mathrm{~N}_{2}(\mathrm{~g})+2 \mathrm{H}_{2}(\mathrm{~g})+1 / 2 \mathrm{Cl}_{2}(\mathrm{~g}) \rightarrow \mathrm{NH}_{4} \mathrm{Cl}(\mathrm{s}) & \Delta \mathrm{H}_{\mathrm{f}}^{\circ}=-314.4 \mathrm{~kJ} / \mathrm{mol} \end{array} \]