Home /
Expert Answers /
Chemistry /
there-are-three-sets-of-sketches-below-showing-the-same-pure-molecular-compound-sulfur-dioxide-m-pa951
(Solved): There are three sets of sketches below, showing the same pure molecular compound (sulfur dioxide, m ...
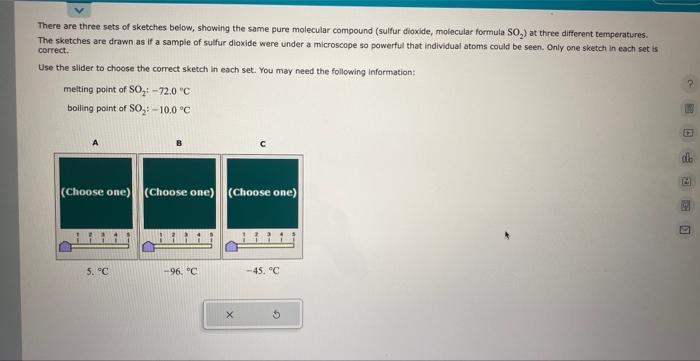
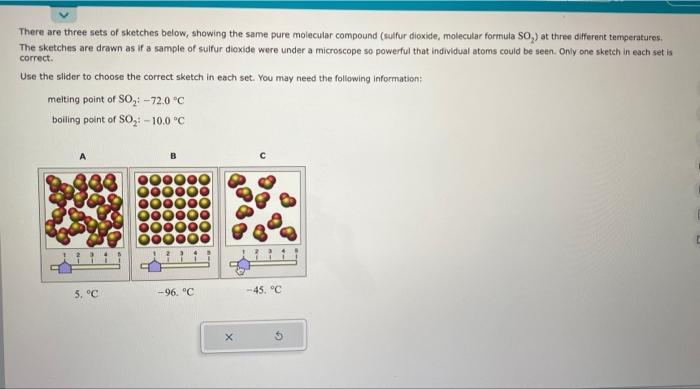
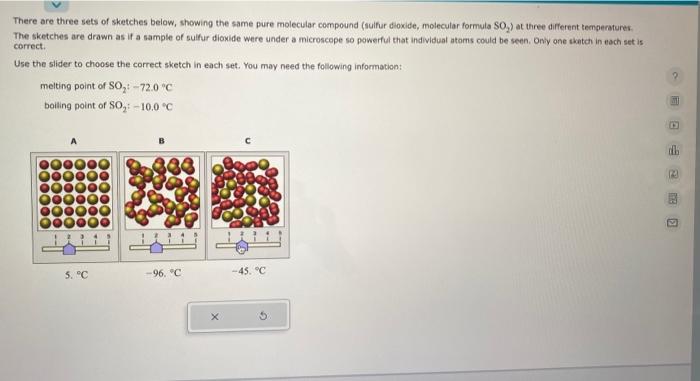
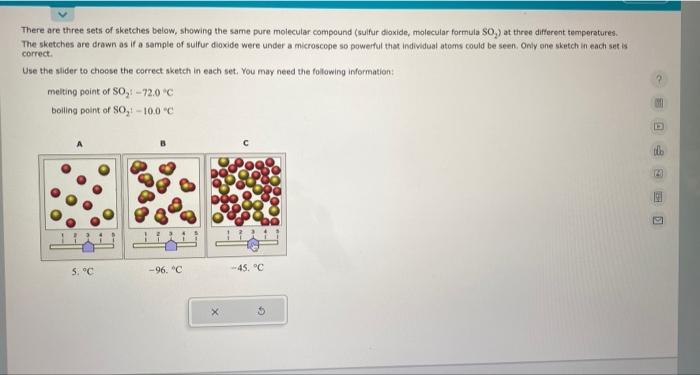
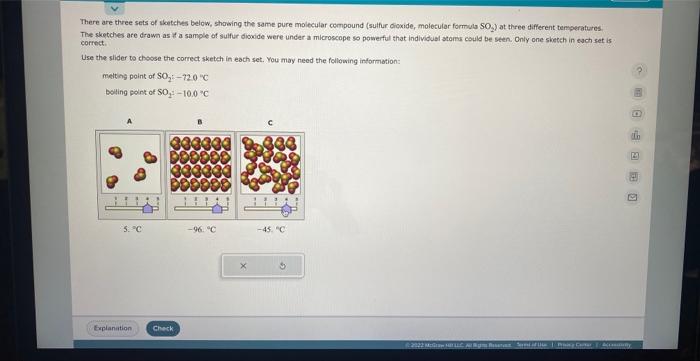
There are three sets of sketches below, showing the same pure molecular compound (sulfur dioxide, molecular formula SO The sketches are drawn as if a sample of sulfur dioxide were under a microscope so powerful that individual atoms could be seen. Only one sketch in each set is correct. Use the slider to choose the correct sketch in each set. You may need the following information: melting point of \( \mathrm{SO}_{2}:-72.0^{\circ} \mathrm{C} \) boiling point of \( \mathrm{SO}_{2}:-10,0^{\circ} \mathrm{C} \)
There are three sets of sketches below, showing the same pure molecular compound (sulfur dioxide, molecular formula SO The sketches are drawn as if a sample of sulfur dioxide were under a microscope so powerful that individual atoms could be seen. Oniy one sketch in each set is correct. Use the slider to choose the correct sketch in each set. You may need the following information: melting point of \( \mathrm{SO}_{2} \div-72.0=\mathrm{C} \) boiling point of \( \mathrm{SO}_{2} ;-10.0^{\circ} \mathrm{C} \)
There are three sets of sketches below, showing the same pure molecilar compound (suifur dioxide, molecular formula SOz) at three different temperatures. The sketches are drawn as if a sample of sulfur dioxide were under a microscope so powerful that individual atoms could be seen. Oniy one tketch in each set is correct: Use the slider to choose the correct sketch in each set. You may need the following information: melting point of \( \mathrm{SO}_{2}+-72.0^{\circ} \mathrm{C} \) boilling point of \( \mathrm{SO}_{2}:-10.0 \div \mathrm{C} \)
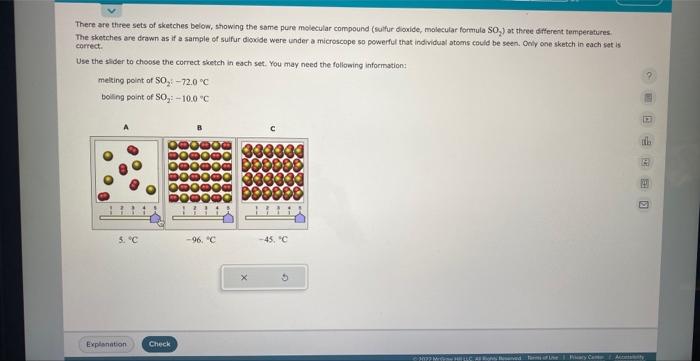
There are three sets of sketches belaw, showing the same pure molecular compound (suifur diaxide, molecular formula SO, ) at three different temperatures. The sketches are drawn as if a sample of sulfur dioxide were under a microscope so powerful that individual atoms could be seen, Oniy one sketch in each set is correct. Use the slider to choose the correct aketch in each set. You may need the folowing information: melting point of \( \mathrm{SO}_{2} 1-72.0 \div \) bolling point of \( \mathrm{SO}_{2} 1-10.0 \mathrm{C} \)
There are thee sets of sketehes below, showing the same pure molecular compound (sulfur cioxide, moletular formulo \( \mathrm{SO} \),) at three different tenperatures: The shootches are drawa as af a sample of sulfur diowide were under a microscope so powerful that indlvidual atomu could be seen. Only one shetch in each set is correct. Use the slider to choose the correct sketch in each set. You may need the following infarmation: metting point of \( \mathrm{SO}_{2}:-120 \mathrm{C} \mathrm{C} \) beiting point of \( \mathrm{SO}_{2}-10.07 \mathrm{C} \)
There are thiee sets of sketches below, showing the same pure molecular compound (eulfur dioxide, molecular formula SO.) at thiree sifferent temperatires. The sketches are drawn as if a sample of suifur diowide were under a microscope so powerful that indwidual atoms could be seen. Oniy one sketch in each set is enerect. Use the shder to choose the correct shetch in each set. You may need the following infocmation meling point of \( \mathrm{SO}_{2},-72.0^{\circ} \mathrm{C} \) boling point of \( \mathrm{SO}_{2}:-10.0 \mathrm{C} \)