Home /
Expert Answers /
Chemistry /
the-voltage-generated-by-the-zinc-concentration-cell-described-by-the-line-notation-mathrm-zn-pa600
(Solved): The voltage generated by the zinc concentration cell described by the line notation \[ \mathrm{Zn} ...
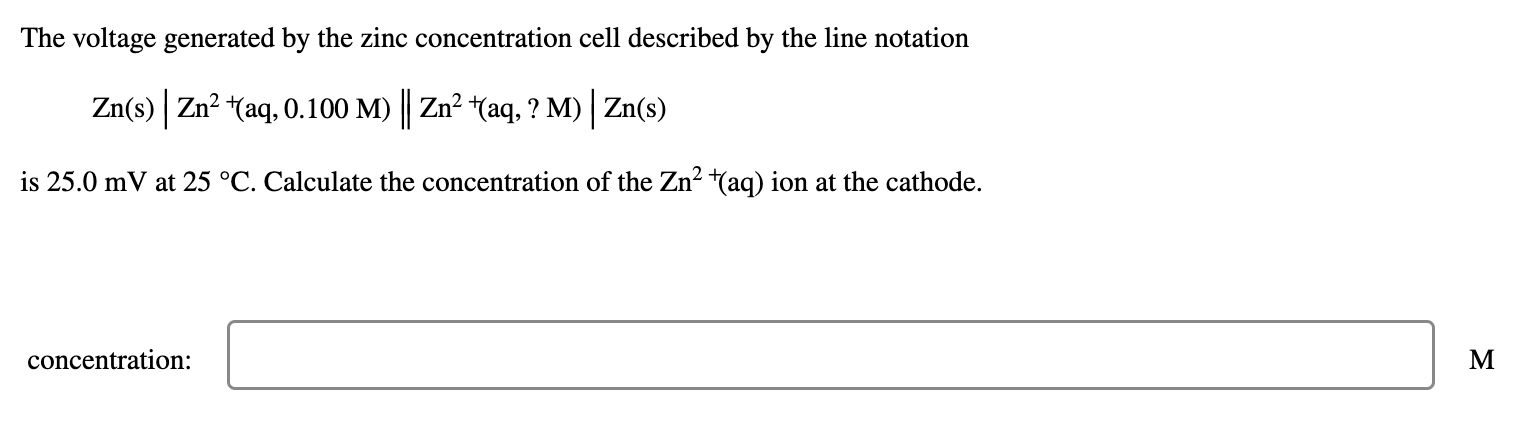
The voltage generated by the zinc concentration cell described by the line notation \[ \mathrm{Zn}(\mathrm{s})\left|\mathrm{Zn}^{2+}{ }^{+}(\mathrm{aq}, 0.100 \mathrm{M}) \| \mathrm{Zn}^{2}{ }^{+}(\mathrm{aq}, ? \mathrm{M})\right| \mathrm{Zn}(\mathrm{s}) \] is \( 25.0 \mathrm{mV} \) at \( 25^{\circ} \mathrm{C} \). Calculate the concentration of the \( \mathrm{Zn}^{2}+(\mathrm{aq}) \) ion at the cathode. concentration: