Home /
Expert Answers /
Chemistry /
the-vapor-pressure-of-liquid-acetone-ch3coch3-is-100-mm-hg-at-281-k-a-0-158-g-sample-of-liquid-pa136
(Solved): The vapor pressure of liquid acetone, CH3COCH3, is 100. mm Hg at 281 K. A 0.158 g sample of liquid ...
The vapor pressure of liquid acetone, CH3COCH3, is 100. mm Hg at 281 K. A 0.158 g sample of liquid CH3COCH 3 is placed in a closed, evacuated 370. mL container at a temperature of 281 K. Assuming that the temperature remains constant, will all of the liquid evaporate? What will the pressure in the container be when equilibrium is reached? mm Hg Submit Answer Retry Entire Group 3 more group attempts remaining
The vapor pressure of liquid ethane thiol, C?H5SH, is 100. mm Hg at 260 K. A sample of C?H5SH is placed in a closed, evacuated 597 mL container at a temperature of 260 K. It is found that all of the C?H5SH is in the vapor phase and that the pressure is 63.0 mm Hg. If the volume of the container is reduced to 388 mL at constant temperature, which of the following statements are correct? Choose all that apply. The pressure in the container will be 100. mm Hg. Only ethane thiol vapor will be present. Liquid ethane thiol will be present. Some of the vapor initially present will condense. No condensation will occur. Submit Answer Retry Entire Group 3 more group attempts remaining
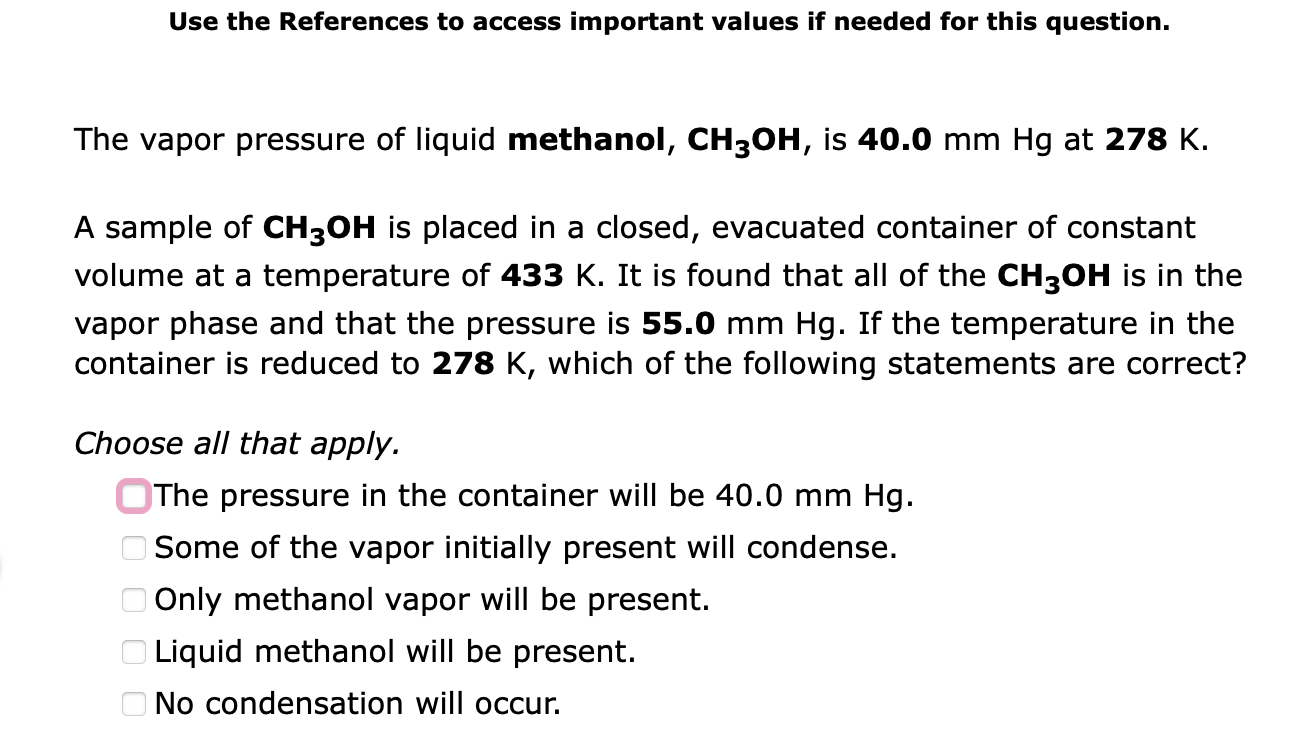
Use the References to access important values if needed for this question. The vapor pressure of liquid methanol, CH3OH, is 40.0 mm Hg at 278 K. A sample of CH3OH is placed in a closed, evacuated container of constant volume at a temperature of 433 K. It is found that all of the CH3OH is in the vapor phase and that the pressure is 55.0 mm Hg. If the temperature in the container is reduced to 278 K, which of the following statements are correct? Choose all that apply. The pressure in the container will be 40.0 mm Hg. Some of the vapor initially present will condense. Only methanol vapor will be present. Liquid methanol will be present. No condensation will occur.