Home /
Expert Answers /
Chemistry /
the-value-of-ka-for-formic-acid-hcooh-is-1-80104-write-the-equation-for-the-reaction-tha-pa977
(Solved): The value of Ka for formic acid, HCOOH, is 1.80104. Write the equation for the reaction tha ...

The value of for formic acid, , is . Write the equation for the reaction that goes with this equilibrium constant. (Use instead of .)
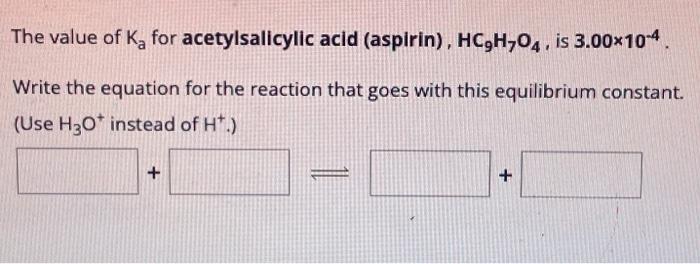
The value of for acetylsalicylic acid (aspirin), , is . Write the equation for the reaction that goes with this equilibrium constant. (Use instead of .)