Home /
Expert Answers /
Mechanical Engineering /
the-unit-cell-shown-for-bcc-and-fcc-structure-can-be-represented-by-tennis-balls-arranged-in-vario-pa467
(Solved): The unit cell shown for bcc and fcc structure can be represented by tennis balls arranged in vario ...
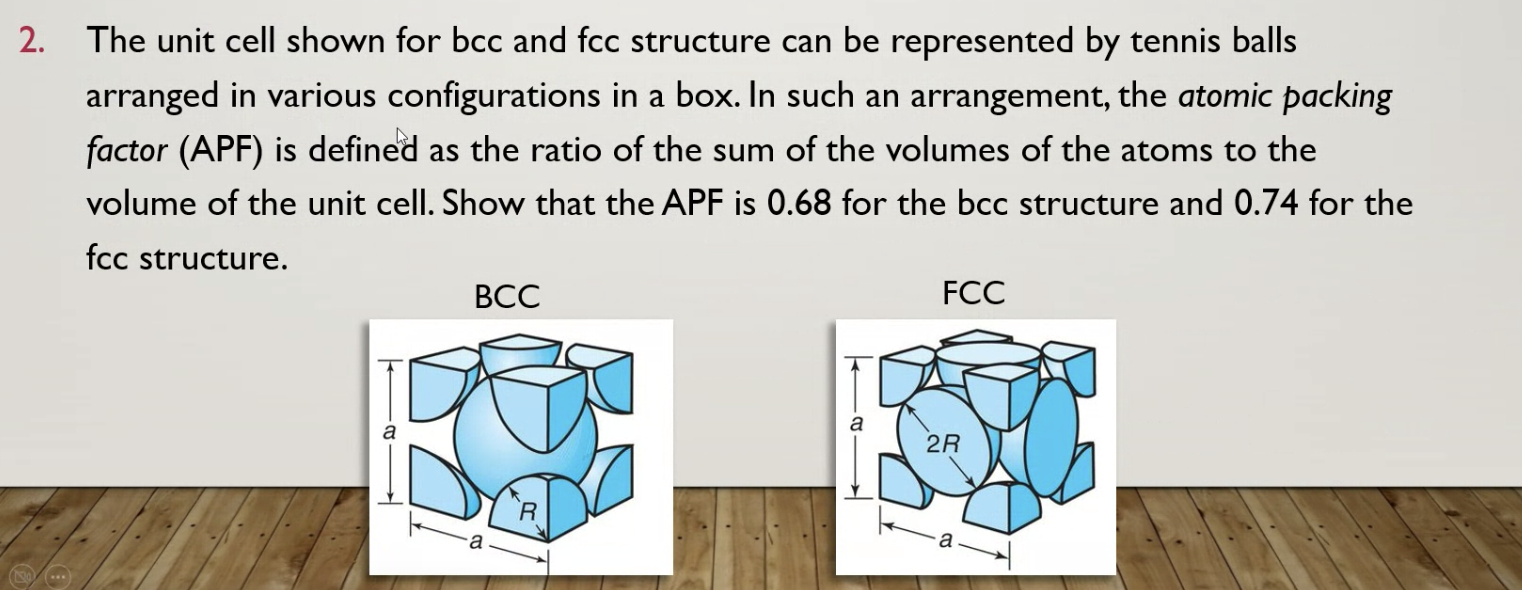
The unit cell shown for bcc and fcc structure can be represented by tennis balls arranged in various configurations in a box. In such an arrangement, the atomic packing factor (APF) is defined as the ratio of the sum of the volumes of the atoms to the volume of the unit cell. Show that the APF is \( 0.68 \) for the bcc structure and \( 0.74 \) for the fcc structure.
Expert Answer
i) For bcc (body centered cubic) : In Body centred cubic structure, there is an atom at the center of the cube. As given : Side of the cube : a radius