Home /
Expert Answers /
Chemical Engineering /
the-synthesis-of-ethyl-chloride-is-accomplished-by-reacting-ethylene-with-hydrogen-chloride-in-the-pa163
(Solved): The synthesis of ethyl chloride is accomplished by reacting ethylene with hydrogen chloride in the ...
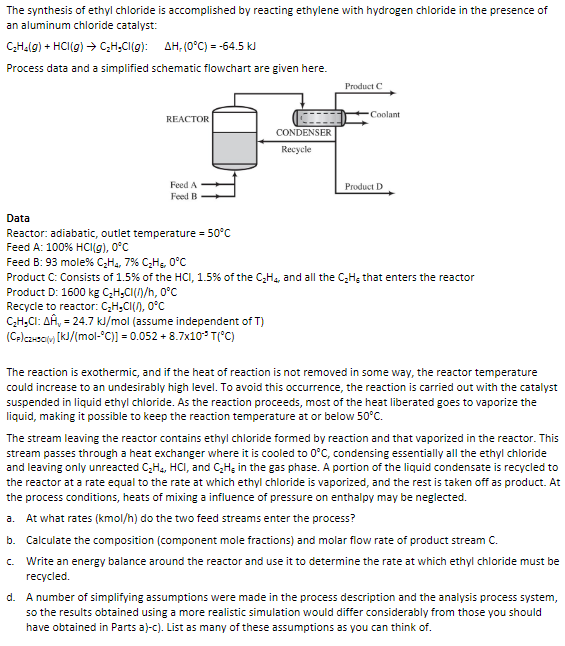
The synthesis of ethyl chloride is accomplished by reacting ethylene with hydrogen chloride in the presence of an aluminum chloride catalyst: \( \mathrm{C}_{2} \mathrm{H}_{4}(g)+\mathrm{HCl}(g) \rightarrow \mathrm{C}_{2} \mathrm{H}_{9} \mathrm{Cl}(g): \quad \Delta \mathrm{H}_{r}\left(0^{\circ} \mathrm{C}\right)=-64.5 \mathrm{~kJ} \) Process data and a simplified schematic flowchart are given here. Data Reactor: adiabatic, outlet temperature \( =50^{\circ} \mathrm{C} \) Feed A: \( 100 \% \mathrm{HCl}(g), 0^{\circ} \mathrm{C} \) Feed B: 93 mole \( \% \mathrm{C}_{2} \mathrm{H}_{4}, 7 \% \mathrm{C}_{2} \mathrm{H}_{6}, 0^{\circ} \mathrm{C} \) Product \( \mathrm{C} \) : Consists of \( 1.5 \% \) of the \( \mathrm{HCl}_{,} 1.5 \% \) of the \( \mathrm{C}_{2} \mathrm{H}_{4} \), and all the \( \mathrm{C}_{2} \mathrm{H}_{5} \) that enters the reactor Product D: \( 1600 \mathrm{~kg} \mathrm{C}_{2} \mathrm{H}_{9} \mathrm{Cl}(f) / \mathrm{h}, 0^{\circ} \mathrm{C} \) Recycle to reactor: \( \mathrm{C}_{2} \mathrm{H}_{9} \mathrm{Cl}\left(\Omega^{\prime}, 0^{\circ} \mathrm{C}\right. \) \( \mathrm{C}_{2} \mathrm{H}_{9} \mathrm{Cl}: \Delta \hat{\mathrm{H}}_{\mathrm{v}}=24.7 \mathrm{~kJ} / \mathrm{mol} \) (assume independent of T) \( \left(\mathrm{C}_{\mathrm{F}}\right)_{\mathrm{cz-so}(\mathrm{v})}\left[\mathrm{kJ} /\left(\mathrm{mol}^{\circ} \mathrm{C}\right)\right]=0.052+8.7 \times 10^{-5} \mathrm{~T}\left({ }^{\circ} \mathrm{C}\right) \) The reaction is exothermic, and if the heat of reaction is not removed in some way, the reactor temperature could increase to an undesirably high level. To avoid this occurrence, the reaction is carried out with the catalyst suspended in liquid ethyl chloride. As the reaction proceeds, most of the heat liberated goes to vaporize the liquid, making it possible to keep the reaction temperature at or below \( 50^{\circ} \mathrm{C} \). The stream leaving the reactor contains ethyl chloride formed by reaction and that vaporized in the reactor. This stream passes through a heat exchanger where it is cooled to \( 0^{\circ} \mathrm{C} \), condensing essentially all the ethyl chloride and leaving only unreacted \( \mathrm{C}_{2} \mathrm{H}_{4}, \mathrm{HCl}_{\text {, and }} \mathrm{C}_{2} \mathrm{H}_{5} \) in the gas phase. A portion of the liquid condensate is recycled to the reactor at a rate equal to the rate at which ethyl chloride is vaporized, and the rest is taken off as product. At the process conditions, heats of mixing a influence of pressure on enthalpy may be neglected. a. At what rates \( (\mathrm{kmol} / \mathrm{h}) \) do the two feed streams enter the process? b. Calculate the composition (component mole fractions) and molar flow rate of product stream C. c. Write an energy balance around the reactor and use it to determine the rate at which ethyl chloride must be recycled. d. A number of simplifying assumptions were made in the process description and the analysis process system, so the results obtained using a more realistic simulation would differ considerably from those you should have obtained in Parts a)-c). List as many of these assumptions as you can think of.