Home /
Expert Answers /
Chemistry /
the-stopcock-connecting-a-1-00l-bulb-containing-carbon-dioxide-gas-at-a-pressure-of-570-mmhg-pa117
(Solved): The stopcock connecting a 1.00L bulb containing carbon dioxide gas at a pressure of 570. mmHg, ...

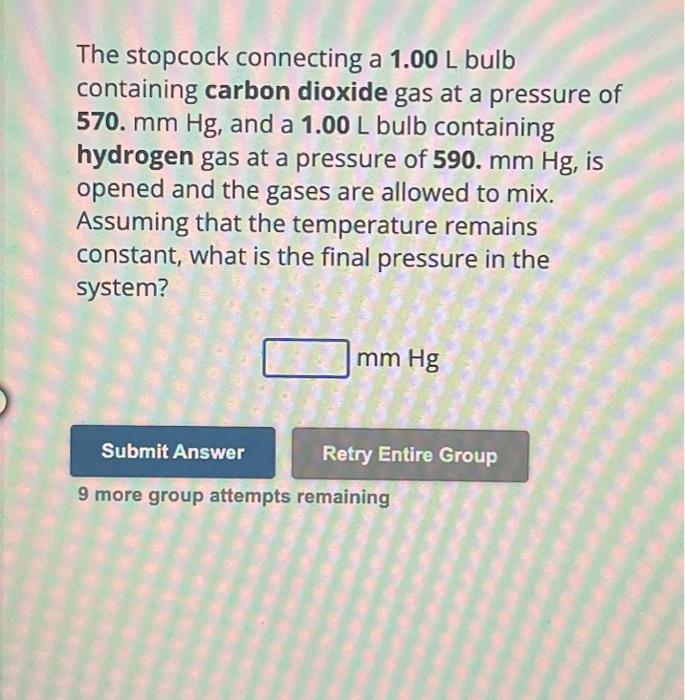

The stopcock connecting a bulb containing carbon dioxide gas at a pressure of 570. , and a bulb containing hydrogen gas at a pressure of , is opened and the gases are allowed to mix. Assuming that the temperature remains constant, what is the final pressure in the system? 9 more group attempts remaining
Zinc metal reacts with excess hydrochloric acid to produce hydrogen gas according to the following equation: In an experiment, the gas formed is collected over water in a flask where the total pressure is and the temperature is , at which temperature the vapor pressure of water is . (1) What is the partial pressure of in the flask? . (2) If the wet gas occupies a volume of , how many moles of are formed? 9 more group attempts remaining
A mixture of methane and argon gases is maintained in a flask at a temperature of . If the partial pressure of methane is and the partial pressure of argon is , what is the total pressure in the flask? atm 9 more group attempts remaining