Home /
Expert Answers /
Chemistry /
the-steam-reforming-of-methane-occurs-according-to-the-following-chemical-equation-mathrm-ch-pa501
(Solved): The steam reforming of methane occurs according to the following chemical equation: \[ \mathrm{CH}_ ...
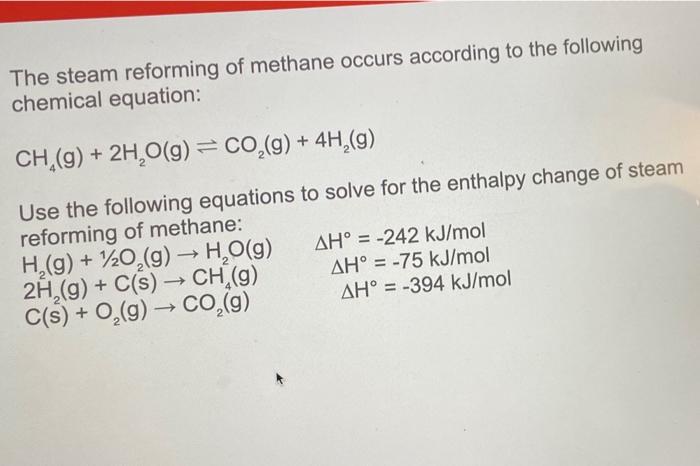
The steam reforming of methane occurs according to the following chemical equation: \[ \mathrm{CH}_{4}(\mathrm{~g})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g}) \rightleftharpoons \mathrm{CO}_{2}(\mathrm{~g})+4 \mathrm{H}_{2}(\mathrm{~g}) \] Use the following equations to solve for the enthalpy change of steam reforming of methane: \( \begin{array}{lr}\mathrm{H}_{2}(\mathrm{~g})+1 / 2 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{H}_{2} \mathrm{O}(\mathrm{g}) & \Delta \mathrm{H}^{\circ}=-242 \mathrm{~kJ} / \mathrm{mol} \\ 2 \mathrm{H}_{2}(\mathrm{~g})+\mathrm{C}(\mathrm{s}) \rightarrow \mathrm{CH}_{4}(\mathrm{~g}) & \Delta \mathrm{H}^{\circ}=-75 \mathrm{~kJ} / \mathrm{mol} \\ \mathrm{C}(\mathrm{s})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{CO}_{2}(\mathrm{~g}) & \Delta \mathrm{H}^{\circ}=-394 \mathrm{~kJ} / \mathrm{mol}\end{array} \)