Home /
Expert Answers /
Chemical Engineering /
the-standard-heat-of-the-combustion-reaction-of-liquid-n-hexane-to-form-co2-g-and-h2o-l-pa629
(Solved): The standard heat of the combustion reaction of liquid n-hexane to form CO2(g) and H2O(l), ...
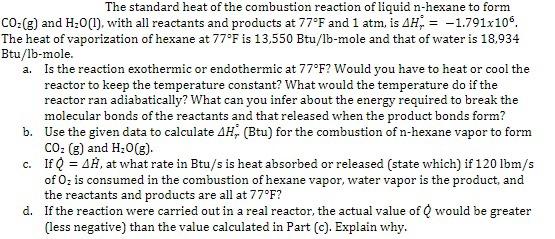
The standard heat of the combustion reaction of liquid -hexane to form and , with all reactants and products at and 1 atm, is . The heat of vaporization of hexane at is and that of water is 18,934 Btu/lb-mole. a. Is the reaction exothermic or endothermic at ? Would you have to heat or cool the reactor to keep the temperature constant? What would the temperature do if the reactor ran adiabatically? What can you infer about the energy required to break the molecular bonds of the reactants and that released when the product bonds form? b. Use the given data to calculate (Btu) for the combustion of -hexane vapor to form and . c. If , at what rate in Btu/s is heat absorbed or released (state which) if of is consumed in the combustion of hexane vapor, water vapor is the product, and the reactants and products are all at ? d. If the reaction were carried out in a real reactor, the actual value of would be greater (less negative) than the value calculated in Part (c). Explain why.
Expert Answer
Standard heat of combustion (AH): The given standa...