Home /
Expert Answers /
Chemical Engineering /
the-saponification-reaction-of-ethyl-acetate-is-a-2-text-at-order-elementary-reversibl-pa133
(Solved): The saponification reaction of ethyl acetate is a \( 2^{\text {at }} \) order, elementary reversibl ...
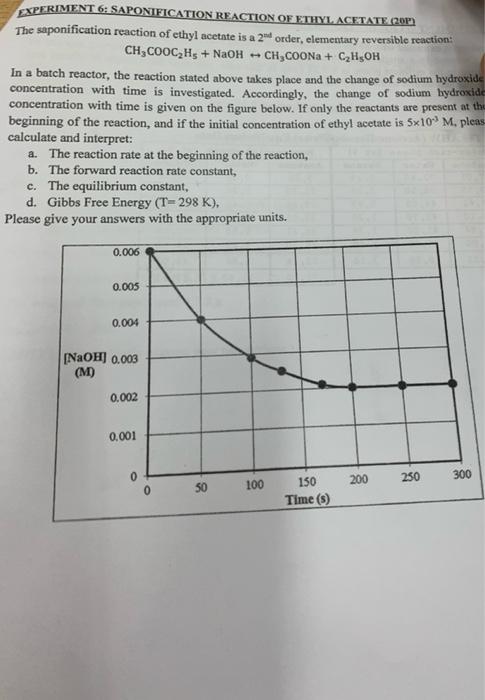
The saponification reaction of ethyl acetate is a \( 2^{\text {at }} \) order, elementary reversible reaction: \[ \mathrm{CH}_{3} \mathrm{COOC}_{2} \mathrm{H}_{5}+\mathrm{NaOH} \leftrightarrow \mathrm{CH}_{3} \mathrm{COONa}+\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH} \] In a batch reactor, the reaction stated above takes place and the change of sodium hydroxide concentration with time is investigated. Accordingly, the change of sodium bydroxide concentration with time is given on the figure below. If only the reactants are present at thi beginning of the reaction, and if the initial concentration of ethyl acetate is \( 5 \times 10^{-3} \mathrm{M} \), pleas calculate and interpret: a. The reaction rate at the beginning of the reaction, b. The forward reaction rate constant, c. The equilibrium constant, d. Gibbs Free Energy \( (\mathrm{T}=298 \mathrm{~K}) \), Please give your answers with the appropriate units.