Home /
Expert Answers /
Chemistry /
the-reduction-of-butanoic-acid-can-form-butanal-and-butan-1-ol-the-boiling-points-of-these-compoun-pa879
(Solved): The reduction of butanoic acid can form butanal and butan-1-ol. The boiling points of these compoun ...
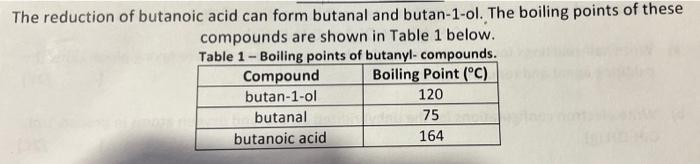

The reduction of butanoic acid can form butanal and butan-1-ol. The boiling points of these compounds are shown in Table 1 below. Table 1-Boiling points of butanyl- compounds. Boiling Point (°C) Compound butan-1-ol 120 75 164 bir butanal butanoic acid
f) 15.00 mL of 0.200 mol/L butanoic acid is titrated with 12.50 mL of NaOH until the equivalence point is reached. Determine the pH of the final titrated solution. (Ka = 1.51 x 10¹5) /4T] g) A series of reduction experiments was carried out at a given temperature. The results are summarized in Table 2 below. Determine the rate law expression for this reaction. [ /2T] Table 2- Experimental rate measurements [R] (mol L-¹) [Butan-1-ol] (mol L-¹) Rate (mol L¹ s¹) 0.100 0.100 2.40 x 10² 0.100 0.200 4.80 x 10² 0.200 0.200 9.60 x 10² h) Below are two proposed reaction mechanisms for this reaction. Explain which reaction mechanism is correct. [/2A] (Let BOH = butan-1-ol; BA = butanal; BH = butanoic acid, R = reducing agent) Mechanism 1 Mechanism 2 BH +R BA (slow) BH + R ? BA BA+R BOH (fast) BA+R BOH (fast) (slow)