Home /
Expert Answers /
Chemistry /
the-reaction-of-bromomethane-with-sodium-hydroxide-in-water-forms-methanol-via-the-reaction-a-ch-pa948
(Solved): The reaction of bromomethane with sodium hydroxide in water forms methanol via the reaction: (a) CH ...
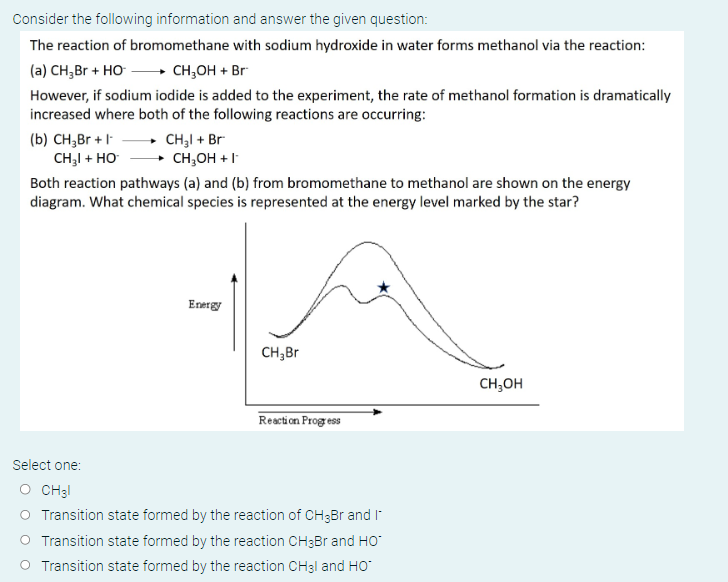
The reaction of bromomethane with sodium hydroxide in water forms methanol via the reaction: (a) However, if sodium iodide is added to the experiment, the rate of methanol formation is dramatically increased where both of the following reactions are occurring: (b) Both reaction pathways (a) and (b) from bromomethane to methanol are shown on the energy diagram. What chemical species is represented at the energy level marked by the star? Select one: Transition state formed by the reaction of and Transition state formed by the reaction and Transition state formed by the reaction and
Expert Answer
The chemical species represented at the energy level marked by the star is the transition state formed by the reaction of The transition state is the highest energy point on the reaction coordinate, and it represents the unstable intermediate stat...