Home /
Expert Answers /
Chemistry /
the-reaction-for-the-metabolism-of-sucrose-mathrm-c-12-mathrm-h-22-mathrm-o-11-pa283
(Solved): The reaction for the metabolism of sucrose, \( \mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11} \), ...
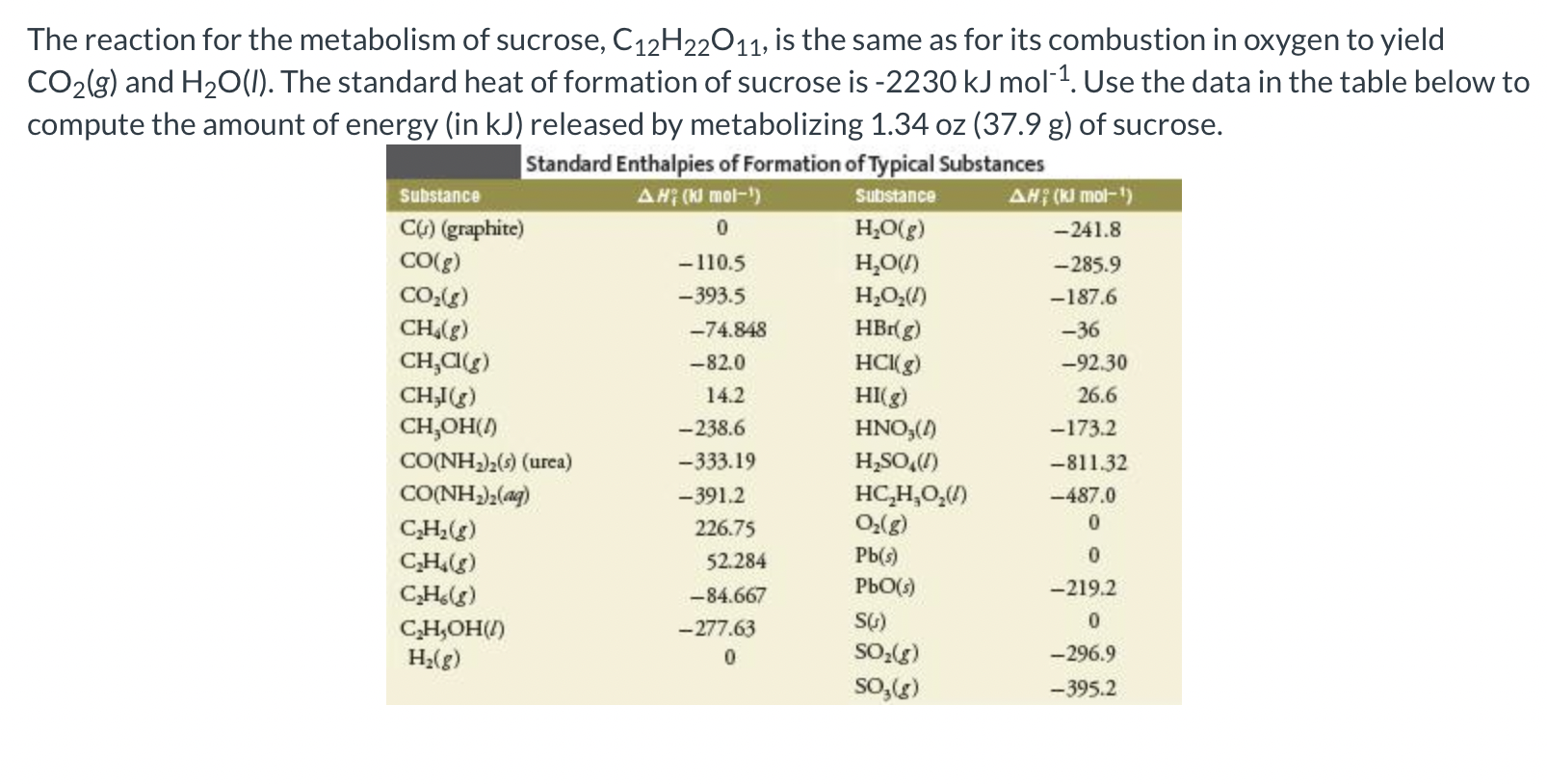
The reaction for the metabolism of sucrose, \( \mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11} \), is the same as for its combustion in oxygen to yield \( \mathrm{CO}_{2}(g) \) and \( \mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \). The standard heat of formation of sucrose is \( -2230 \mathrm{~kJ} \mathrm{~mol}^{-1} \). Use the data in the table below to compute the amount of energy (in \( \mathrm{kJ}) \) released by metabolizing \( 1.34 \mathrm{oz}(37.9 \mathrm{~g}) \) of sucrose.