Home /
Expert Answers /
Chemical Engineering /
the-reaction-ch3ch3-gt-ch2ch2-h2-can-have-the-following-mechanism-ch3ch3-x-pa358
(Solved): The reaction CH3CH3>CH2CH2+H2 can have the following mechanism CH3CH3+X ...
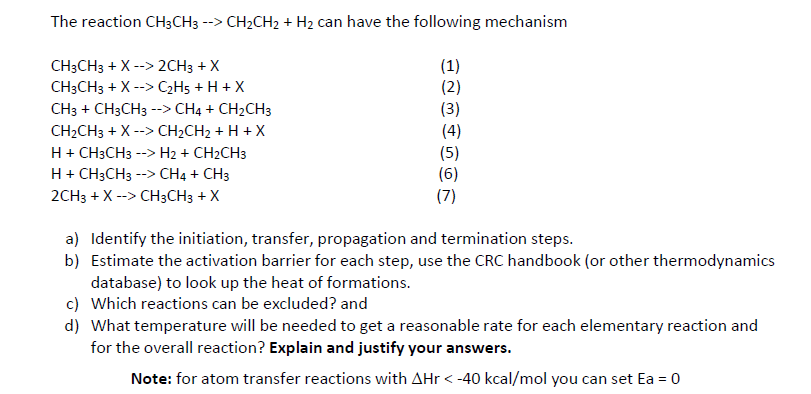
The reaction can have the following mechanism (3) (6) a) Identify the initiation, transfer, propagation and termination steps. b) Estimate the activation barrier for each step, use the CRC handbook (or other thermodynamics database) to look up the heat of formations. c) Which reactions can be excluded? and d) What temperature will be needed to get a reasonable rate for each elementary reaction and for the overall reaction? Explain and justify your answers. Note: for atom transfer reactions with you can set