Home /
Expert Answers /
Chemistry /
the-reaction-between-persulphate-ions-and-iodide-ions-is-very-slow-in-the-absence-of-a-catalyst-it-pa641
(Solved): The reaction between persulphate ions and iodide ions is very slow in the absence of a catalyst. It ...
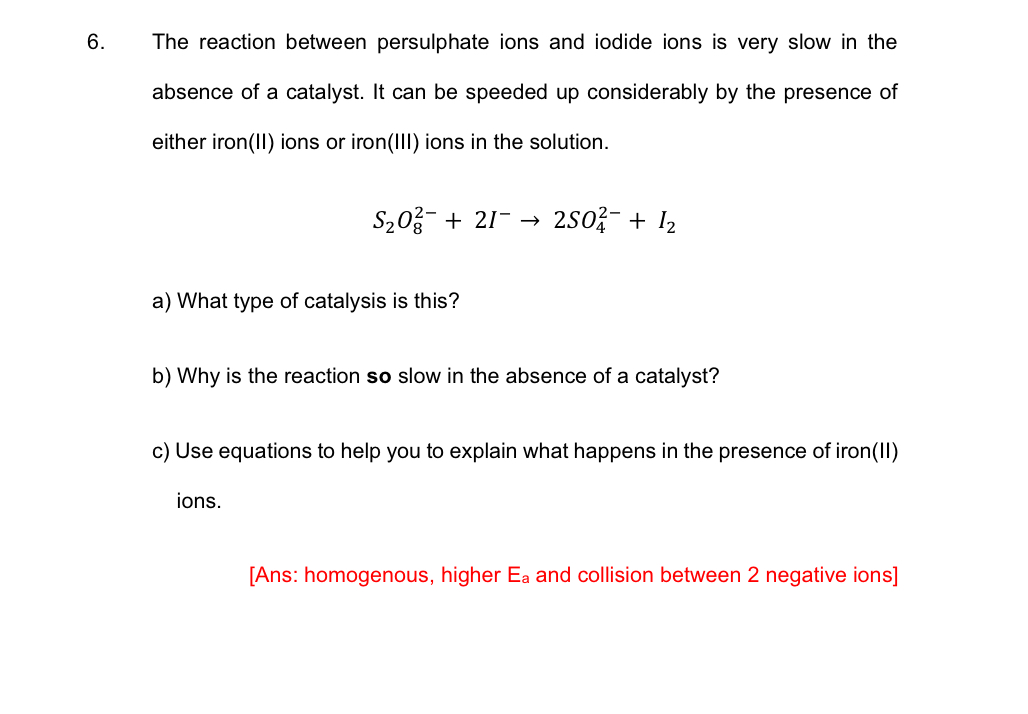
The reaction between persulphate ions and iodide ions is very slow in the absence of a catalyst. It can be speeded up considerably by the presence of either iron(II) ions or iron(III) ions in the solution.
S_(2)O_(8)^(2-)+2I^(-)->2SO_(4)^(2-)+I_(2)a) What type of catalysis is this? b) Why is the reaction so slow in the absence of a catalyst? c) Use equations to help you to explain what happens in the presence of iron(II) ions. [Ans: homogenous, higher
E_(a)and collision between 2 negative ions]