Home /
Expert Answers /
Chemistry /
the-reaction-between-ammonia-and-oxygen-is-given-below-2-mathrm-nh-3-g-2-mathrm-o-2-g-pa881
(Solved): The reaction between ammonia and oxygen is given below: \[ 2 \mathrm{NH}_{3}(g)+2 \mathrm{O}_{2}(g) ...
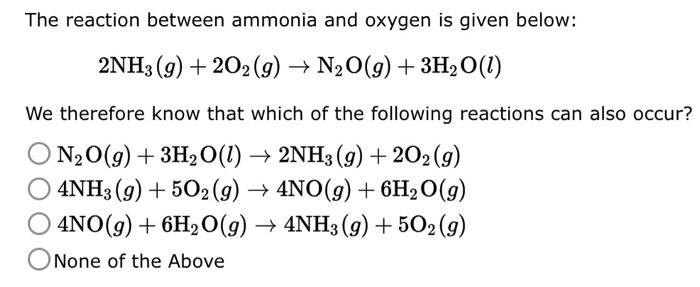
The reaction between ammonia and oxygen is given below: \[ 2 \mathrm{NH}_{3}(g)+2 \mathrm{O}_{2}(g) \rightarrow \mathrm{N}_{2} \mathrm{O}(g)+3 \mathrm{H}_{2} \mathrm{O}(l) \] We therefore know that which of the following reactions can also occur? \[ \begin{array}{l} \mathrm{N}_{2} \mathrm{O}(g)+3 \mathrm{H}_{2} \mathrm{O}(l) \rightarrow 2 \mathrm{NH}_{3}(g)+2 \mathrm{O}_{2}(g) \\ 4 \mathrm{NH}_{3}(g)+5 \mathrm{O}_{2}(g) \rightarrow 4 \mathrm{NO}(g)+6 \mathrm{H}_{2} \mathrm{O}(g) \\ 4 \mathrm{NO}(g)+6 \mathrm{H}_{2} \mathrm{O}(g) \rightarrow 4 \mathrm{NH}_{3}(g)+5 \mathrm{O}_{2}(g) \end{array} \] None of the Above
Expert Answer
N2O(g) + 3 H2O(l) --> 2 NH3(g) +2O2(g) Reason : Given reaction:- 2 NH3(g) + 2 O2(g) --> N2O(g) +3H2O(l) This means, when two molecules of NH3 reacts it produces 2 mo