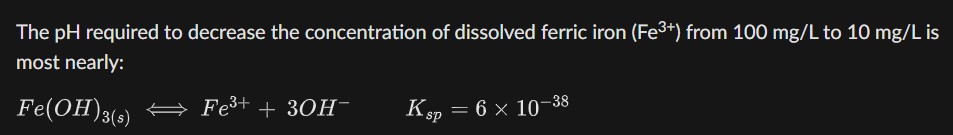Home /
Expert Answers /
Chemistry /
the-ph-required-to-decrease-the-concentration-of-dissolved-ferric-iron-fe-3-from-100m-g-l-to-pa455
(Solved): The pH required to decrease the concentration of dissolved ferric iron (Fe^(3+)) from 100m(g)/(L) to ...
The pH required to decrease the concentration of dissolved ferric iron (Fe^(3+)) from 100m(g)/(L) to 10m(g)/(L) is
most nearly:
Fe(OH)_(3(s))LongleftrightarrowFe^(3+)+3OH^(-),K_(sp)=6\times 10^(-38)