Home /
Expert Answers /
Chemistry /
the-oxidation-of-iodide-ion-by-hydrogen-peroxide-in-an-acidic-solution-is-described-by-the-balanced-pa543
(Solved): The oxidation of iodide ion by hydrogen peroxide in an acidic solution is described by the balanced ...
The oxidation of iodide ion by hydrogen peroxide in an acidic solution is described by the balanced equation \[ \mathrm{H}_{2} \mathrm{O}_{2}(a q)+3 \mathrm{I}^{-}(a q)+2 \mathrm{H}^{+}(a q) \rightarrow \mathrm{I}_{3}^{-}(a q)+2 \mathrm{H}_{2} \mathrm{O}(l) \] The rate of formation of the red triiodide ion, \( \Delta\left[\mathrm{I}_{3}^{-}\right] / \Delta t \), can be determined by measuring the rate of appearance of the color (see the figure). Part A What is the rate law for the formation of \( \mathrm{I}_{3}^{-} \)? \[ \begin{array}{l} \text { Rate }=k\left[\mathrm{H}_{2} \mathrm{O}_{2}\right]\left[\mathrm{I}^{-}\right] \\ \text {Rate }=k\left[\mathrm{H}_{2} \mathrm{O}_{2}\right]^{2}\left[\mathrm{I}^{-}\right]^{2} \\ \text { Rate }=k\left[\mathrm{I}^{-}\right] \\ \text {Rate }=k\left[\mathrm{H}_{2} \mathrm{O}_{2}\right] \end{array} \]
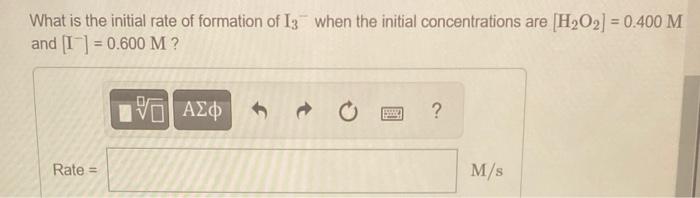
What is the initial rate of formation of \( \mathrm{I}_{3}{ }^{-} \)when the initial concentrations are \( \left[\mathrm{H}_{2} \mathrm{O}_{2}\right]=0.400 \mathrm{M} \) and \( \left[\mathrm{I}^{-}\right]=0.600 \mathrm{M} \) ?
Expert Answer
Rate law for the formation of I3- can be determined