Home /
Expert Answers /
Chemistry /
the-mole-concept-chemical-formula-of-a-hydrate-lab-report-exercise-1-water-of-hydration-data-ta-pa267
(Solved): The Mole Concept: Chemical Formula of a Hydrate - Lab Report Exercise 1: Water of Hydration Data Ta ...
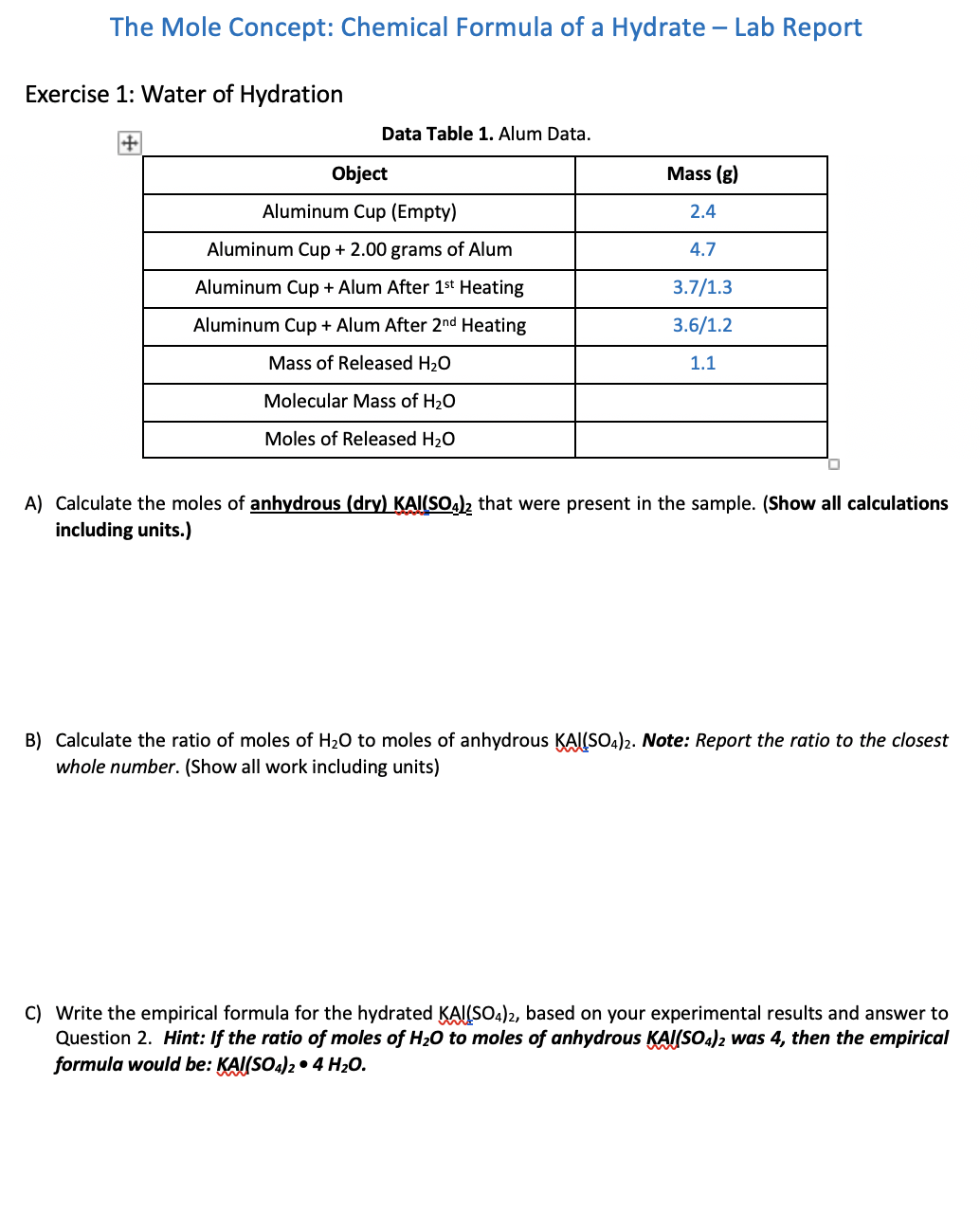
The Mole Concept: Chemical Formula of a Hydrate - Lab Report Exercise 1: Water of Hydration Data Table 1. Alum Data. A) Calculate the moles of anhydrous (dry) that were present in the sample. (Show all calculations including units.) B) Calculate the ratio of moles of to moles of anhydrous . Note: Report the ratio to the closest whole number. (Show all work including units) C) Write the empirical formula for the hydrated , based on your experimental results and answer to Question 2. Hint: If the ratio of moles of to moles of anhydrous was 4 , then the empirical formula would be: .
Expert Answer
Ans: We know the molecular formula of alum is Now from the given data, we have