Home /
Expert Answers /
Chemistry /
the-mercury-in-a-1-0451-g-sample-was-precipitated-with-an-excess-of-paraperiodic-acid-h-io-5hg-pa300
(Solved): The mercury in a 1.0451-g sample was precipitated with an excess of paraperiodic acid, H,IO 5Hg+ ...
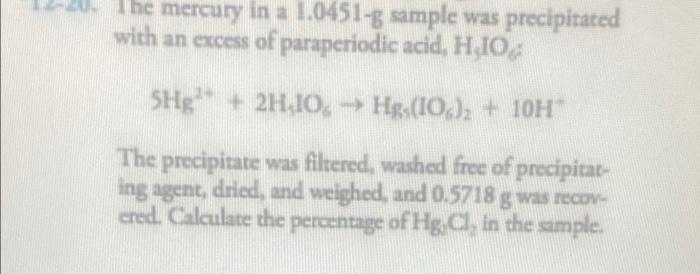
The mercury in a 1.0451-g sample was precipitated with an excess of paraperiodic acid, H,IO 5Hg²+ 2H,IO, Hgs(106)2 + 10H™ The precipitate was filtered, washed free of precipitat- ing agent, dried, and weighed, and 0.5718 g was recov ered. Calculate the percentage of Hg, Cl, in the sample.