Home /
Expert Answers /
Chemical Engineering /
the-law-of-corresponding-states-suggests-that-gases-exhibit-very-similar-non-ideal-behavior-when-ex-pa931
(Solved): The Law of Corresponding States suggests that gases exhibit very similar non-ideal behavior when ex ...
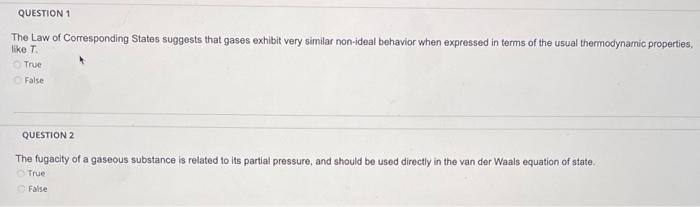
The Law of Corresponding States suggests that gases exhibit very similar non-ideal behavior when expressed in terms of the usual thermodynamic properties, like \( T \) True False QUeSTION 2 The fugacity of a gaseous substance is related to its partial pressure, and should be used directly in the van der Waals equation of state. True False
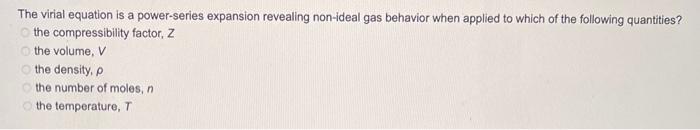
The virial equation is a power-series expansion revealing non-ideal gas behavior when applied to which of the following quantities? the compressibility factor, \( Z \) the volume, \( V \) the density, \( \rho \) the number of moles, \( n \) the temperature, \( T \)
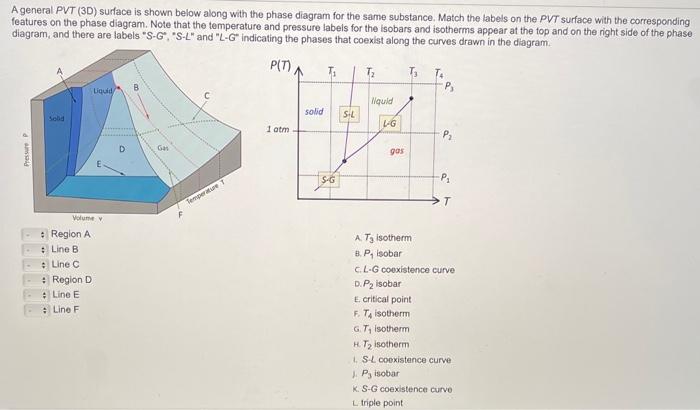
A general PVT (3D) surface is shown below along with the phase diagram for the same substance. Match the labels on the PVT surface with the corresponding features on the phase diagram. Note that the temperature and pressure labeis for the isobars and isotherms appear at the fop and on the right side of the phase diagram, and there are labels "S-G", "S-L" and "L-G" indicating the phases that coexist along the curves drawn in the diagram. Region A A. \( T_{3} \) isotherm Line B B. \( P_{1} \) isobar Line \( C \) c. L-G coexistence curve Region D D. \( P_{2} \) isobar Line \( E \) E. critical point F. \( T_{4} \) isotherm G. \( T_{1} \) isotherm H. \( T_{2} \) isotherm 1. S.L cooxistence curve 1. \( P_{3} \) isobar K. S-G coexistence curvo L triple point
Expert Answer
1) It is TRUE as gases of same behaviour when expressed in thermodynamics intities