Home /
Expert Answers /
Chemistry /
the-ksp-of-silver-bromide-is-5-4-times-10-13-at-298-mathrm-k-what-is-the-de-pa657
(Solved): The Ksp of silver bromide is \( 5.4 \times 10^{-13} \) at \( 298 \mathrm{~K} \). What is the \( \De ...
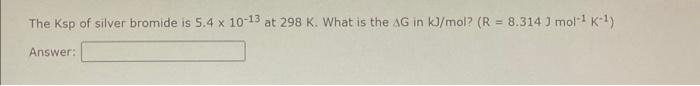
The Ksp of silver bromide is \( 5.4 \times 10^{-13} \) at \( 298 \mathrm{~K} \). What is the \( \Delta \mathrm{G} \) in \( \mathrm{kJ} / \mathrm{mol} \) ? \( \left(\mathrm{R}=8.314 \mathrm{~J} \mathrm{~mol}{ }^{-1} \mathrm{~K}^{-1}\right) \) Answer:
Expert Answer
Given: Ksp of silver bromide = 5.4 x 10-13 Temperature (T) = 298 K R = 8.314 J mol-1 K-1 Find