Home /
Expert Answers /
Chemistry /
the-image-shows-a-plot-of-the-potential-energy-of-2-hydrogen-atoms-vs-the-distance-of-their-nuclei-pa996
(Solved): The image shows a plot of the potential energy of 2 hydrogen atoms vs the distance of their nuclei. ...
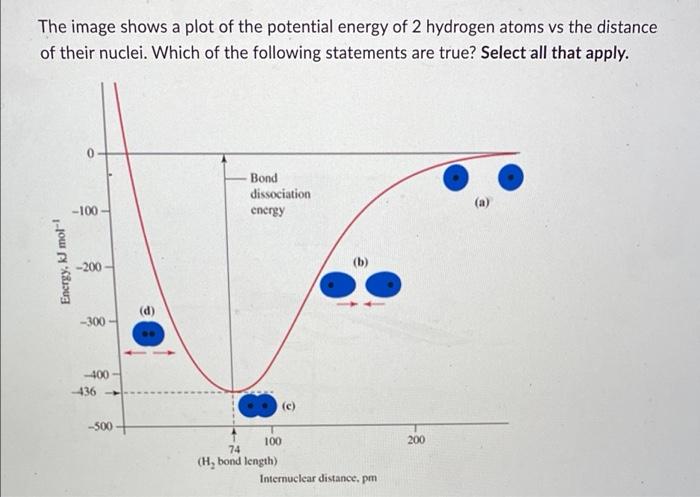

The image shows a plot of the potential energy of 2 hydrogen atoms vs the distance of their nuclei. Which of the following statements are true? Select all that apply. Energy, kJ mol-¹ -100 -200 -300 -400 -436 -500 (d) Bond dissociation energy (c) T 100 74 (H, bond length) Internuclear distance. pm 200 (a)
At an internuclear distance of 5 pm, repulsive forces are greater than attractive forces When two hydrogen atoms that are far apart approach each other, the potential energy initially decreases When the distance between two hydrogen atoms is 74 pm, electron density is redistributed into the internuclear region The potential energy at an internuclear distance of 87 pm is lower than when H? is at its optimal bond length A covalent bond is formed when the potential energy is zero
Expert Answer
#Solution :- The correct statements are :- A. At an internuclear distance