Home /
Expert Answers /
Chemistry /
the-identity-of-an-unknown-monoprotic-organic-acid-is-determined-by-titration-a-0-509-mathrm-pa304
(Solved): The identity of an unknown monoprotic organic acid is determined by titration. A \( 0.509 \mathrm{~ ...
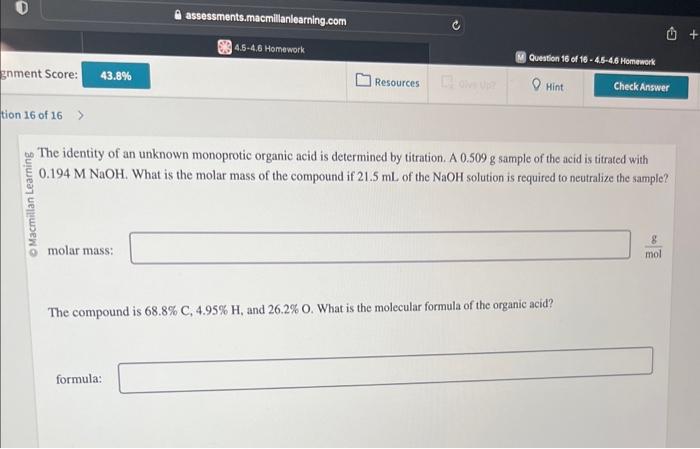
The identity of an unknown monoprotic organic acid is determined by titration. A \( 0.509 \mathrm{~g} \) sample of the acid is titrated with \( 0.194 \mathrm{M} \mathrm{NaOH} \). What is the molar mass of the compound if \( 21.5 \mathrm{~mL} \) of the \( \mathrm{NaOH} \) solution is required to neutralize the sample? molar mass: The compound is \( 68.8 \% \mathrm{C}, 4.95 \% \mathrm{H} \), and \( 26.2 \% \mathrm{O} \). What is the molecular formula of the organic acid? formula: