Home /
Expert Answers /
Chemical Engineering /
the-galvanic-couple-of-mathrm-cu-and-mathrm-fe-makes-the-corrosion-of-cu-more-likel-pa388
(Solved): The galvanic couple of \( \mathrm{Cu} \) and \( \mathrm{Fe} \) makes the corrosion of Cu more likel ...
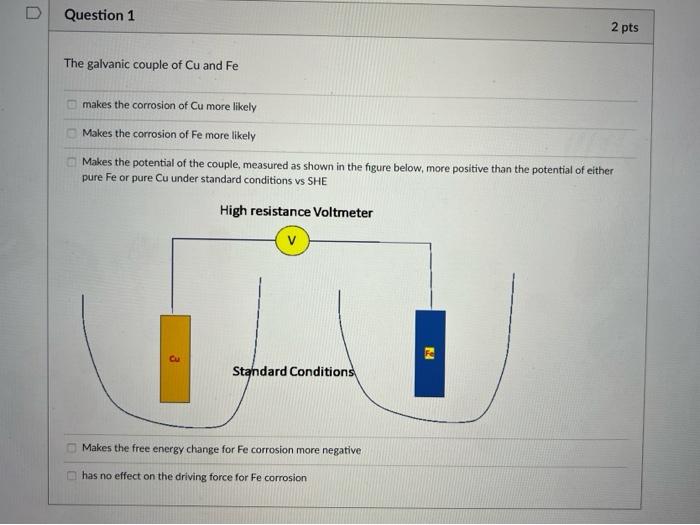
The galvanic couple of \( \mathrm{Cu} \) and \( \mathrm{Fe} \) makes the corrosion of Cu more likely Makes the corrosion of Fe more likely Makes the potential of the couple, measured as shown in the figure below, more positive than the potential of either pure Fe or pure \( \mathrm{Cu} \) under standard conditions vs SHE Makes the free energy change for Fe corrosion more negative has no effect on the driving force for Fe corrosion
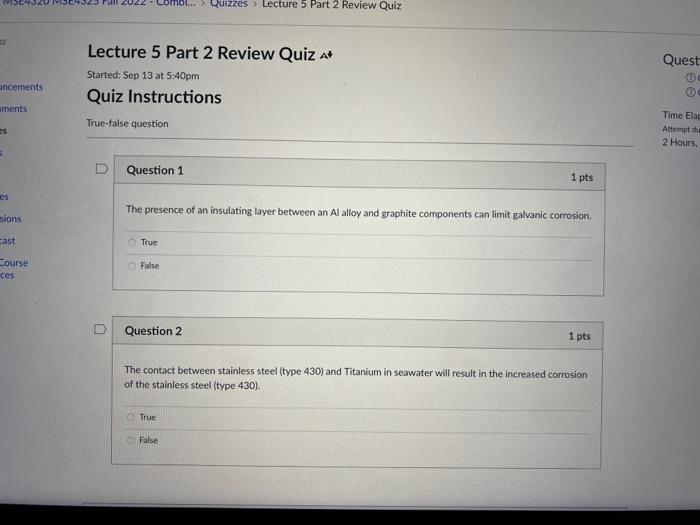
The presence of an insulating layer between an Al alloy and graphite components can limit galvanic corrosion. True False Question 2 1 pts. The contact between stainless steel (type 430\( ) \) and Titanium in seawater will result in the increased corrosion of the stainless steel (type 430). True False
Expert Answer
Quiz questions Question 1:True The presence of an insulating layer between an Al alloy and graphite components can limit galvanic corrosion. True Galvanic corrosion can be prevented by: Selecting materials w