Home /
Expert Answers /
Chemistry /
the-following-thermochemical-equation-is-for-the-reaction-of-mathrm-na-s-with-mathrm-h-pa956
(Solved): The following thermochemical equation is for the reaction of \( \mathrm{Na}(s) \) with \( \mathrm{H ...
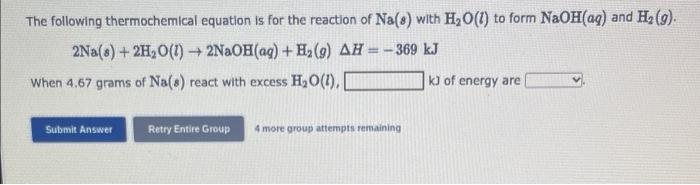
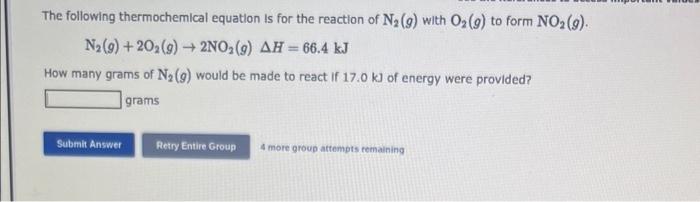
The following thermochemical equation is for the reaction of \( \mathrm{Na}(s) \) with \( \mathrm{H}_{2} \mathrm{O}(l) \) to form \( \mathrm{NaOH}(a q) \) and \( \mathrm{H}_{2}(g) \). \[ 2 \mathrm{Na}(s)+2 \mathrm{H}_{2} \mathrm{O}(l)+2 \mathrm{NaOH}(a q)+\mathrm{H}_{2}(g) \Delta H=-369 \mathrm{~kJ} \] When \( 4.67 \) grams of \( \mathrm{Na}(s) \) react with excess \( \mathrm{H}_{2} \mathrm{O}(l) \), kJ of energy are
The following thermochemical equation is for the reaction of \( \mathrm{N}_{2}(g) \) with \( \mathrm{O}_{2}(g) \) to form \( \mathrm{NO}_{2}(g) \). \[ \mathrm{N}_{2}(g)+2 \mathrm{O}_{2}(g) \rightarrow 2 \mathrm{NO}_{2}(g) \Delta H=66.4 \mathrm{~kJ} \] How many grams of \( \mathrm{N}_{2}(g) \) would be made to react if \( 17.0 \mathrm{~kJ} \) of energy were provided? grams