Home /
Expert Answers /
Chemistry /
the-following-reaction-was-performed-in-a-sealed-vessel-at-718c-h2-g-i2-g-2hi-g-i-pa400
(Solved): The following reaction was performed in a sealed vessel at 718C H2(g)+I2(g)2HI(g) I ...
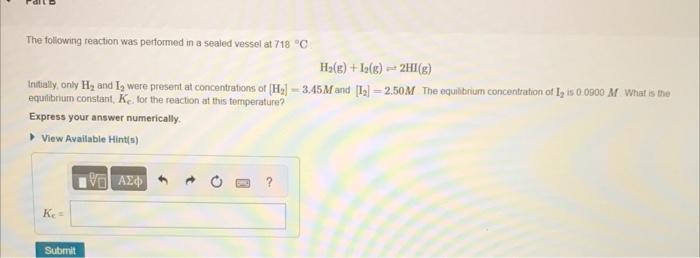
The following reaction was performed in a sealed vessel at Initially, only and were presert at concentrations of and The equilbnium concentration of is . What is me equilibrium constant, , for the reaction at this temperature? Express your answer numerically.