Home /
Expert Answers /
Chemistry /
the-following-reaction-has-a-second-order-rate-law-2-mathrm-no-g-mathrm-cl-2-g-righta-pa433
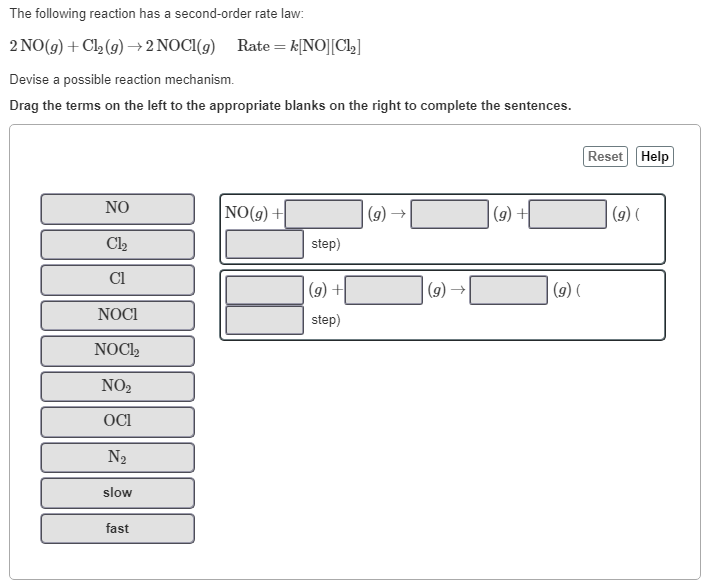
(Solved): The following reaction has a second-order rate law: \[ 2 \mathrm{NO}(g)+\mathrm{Cl}_{2}(g) \righta ...
The following reaction has a second-order rate law: \[ 2 \mathrm{NO}(g)+\mathrm{Cl}_{2}(g) \rightarrow 2 \mathrm{NOCl}(g) \quad \text { Rate }=k[\mathrm{NO}]\left[\mathrm{Cl}_{2}\right] \] Devise a possible reaction mechanism. Drag the terms on the left to the appropriate blanks on the right to complete the sentences. \( \mathrm{NO}(g)+\quad(g) \rightarrow \quad(g)+\quad(g)( \)