Home /
Expert Answers /
Chemical Engineering /
the-following-question-refer-to-the-phase-diagram-shown-here-reprinted-by-permission-of-asm-inter-pa684
(Solved): The following question refer to the phase diagram shown here. Reprinted by permission of ASM Inter ...
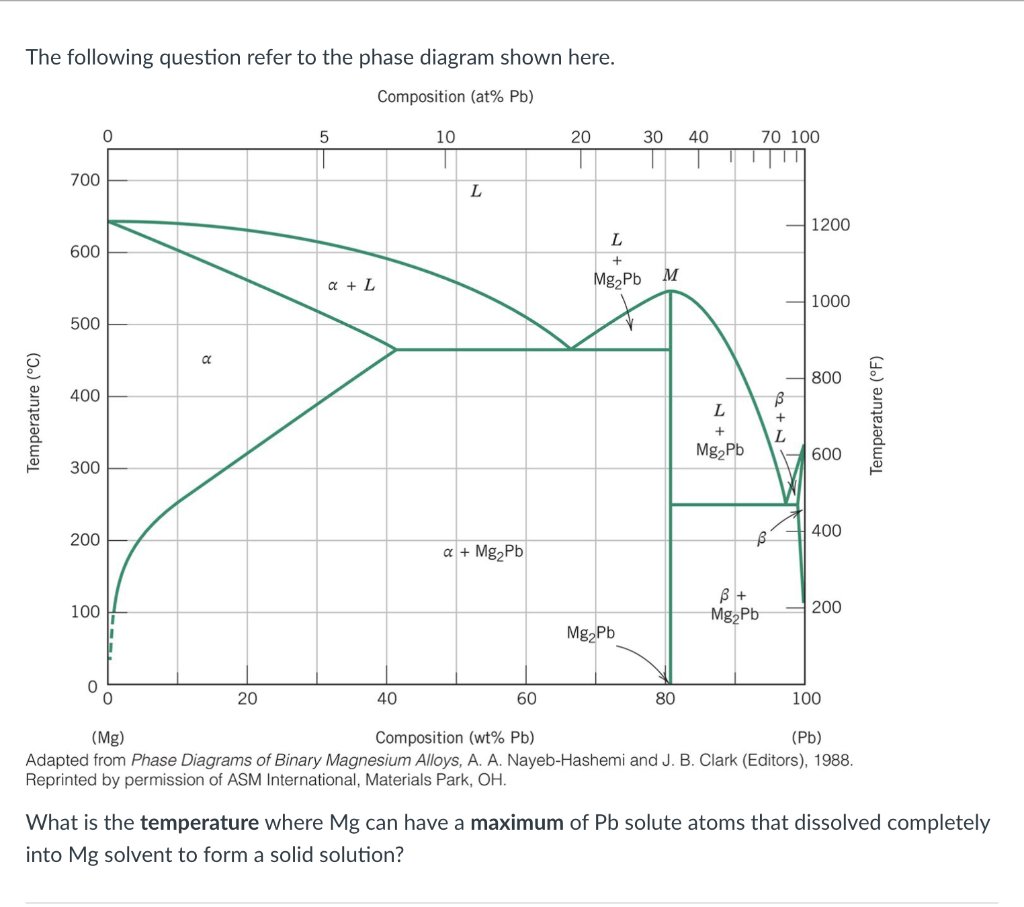
The following question refer to the phase diagram shown here. Reprinted by permission of ASM Internátional, Materials Park, \( \mathrm{OH} \). What is the temperature where \( \mathrm{Mg} \) can have a maximum of \( \mathrm{Pb} \) solute atoms that dissolved completely into \( \mathrm{Mg} \) solvent to form a solid solution?
Room temperature \( 100 \mathrm{C} \) \( 250 \mathrm{C} \) \( 550 \mathrm{C} \) \( 640 \mathrm{C} \) \( 460 \mathrm{C} \) \( 330 \mathrm{C} \)