Home /
Expert Answers /
Chemistry /
the-following-lewis-diagram-represents-the-valence-electron-configuration-of-a-main-group-element-pa647
(Solved): The following Lewis diagram represents the valence electron configuration of a main-group element. ...
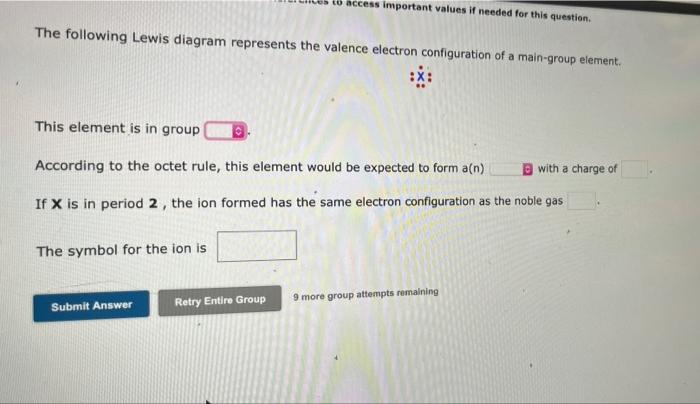
The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group According to the octet rule, this element would be expected to form a(n). If X is in period 2, the ion formed has the same electron configuration as the noble gas The symbol for the ion is to access important values if needed for this question. Submit Answer Retry Entire Group 9 more group attempts remaining with a charge of
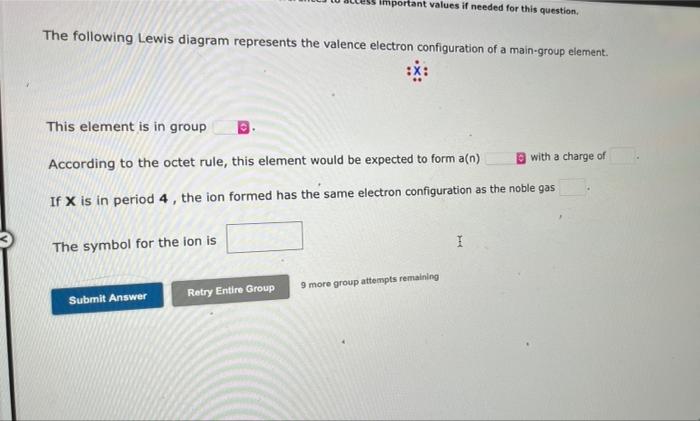
The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group According to the octet rule, this element would be expected to form a(n) If X is in period 4, the ion formed has the same electron configuration as the noble gas The symbol for the ion is important values if needed for this question. Submit Answer Retry Entire Group 9 more group attempts remaining I with a charge of
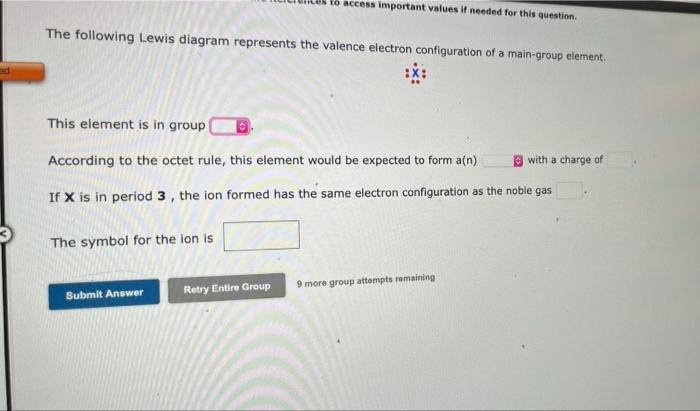
edi The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group According to the octet rule, this element would be expected to form a(n) If X is in period 3, the ion formed has the same electron configuration as the noble gas The symbol for the ion is to access important values if needed for this question. Submit Answer Retry Entire Group 9 more group attempts remaining with a charge of