Home /
Expert Answers /
Chemistry /
the-following-lewis-diagram-represents-the-valence-electron-configuration-of-a-main-group-element-pa488
(Solved): The following Lewis diagram represents the valence electron configuration of a main-group element. ...
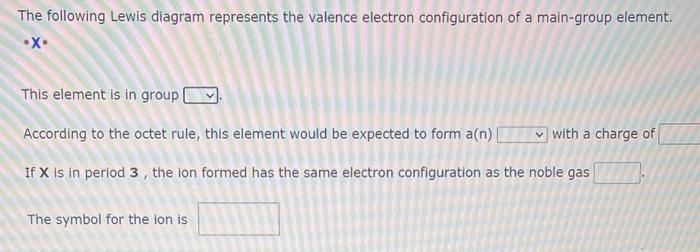
The following Lewis diagram represents the valence electron configuration of a main-group element. - X This element is in group According to the octet rule, this element would be expected to form a(n) with a charge of If \( \mathbf{X} \) is in period \( \mathbf{3} \), the ion formed has the same electron configuration as the noble gas The symbol for the ion is
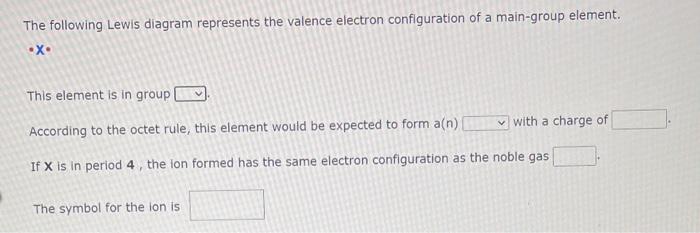
The following Lewis diagram represents the valence electron conflguration of a main-group element. - X• This element is in group According to the octet rule, this element would be expected to form \( a(n) \quad \) with a charge of If \( \mathrm{X} \) is in period 4 , the ion formed has the same electron configuration as the noble gas The symbol for the ion is
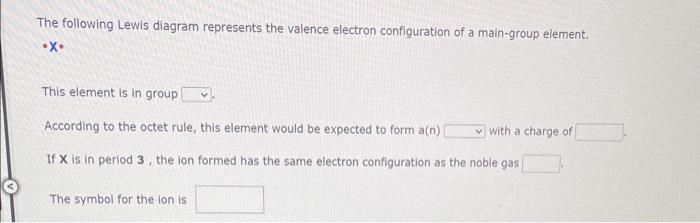
The following Lewis diagram represents the valence electron configuration of a main-group element. - X. This element is in group According to the octet rule, this element would be expected to form a( \( n) \) with a charge of If \( X \) is in period 3 , the lon formed has the same electron configuration as the noble gas The symbol for the lon is
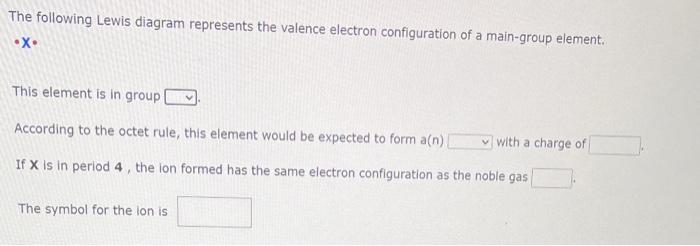
The following Lewis diagram represents the valence electron configuration of a main-group element. X This element is in group According to the octet rule, this element would be expected to form \( a(n) \) with a charge of If \( X \) is in period 4 , the ion formed has the same electron configuration as the noble gas The symbol for the lon is