Home /
Expert Answers /
Earth Sciences /
the-following-equation-represents-the-relation-between-pressure-temperature-and-volume-for-a-spec-pa771
(Solved): The following equation represents the relation between pressure, temperature and volume for a spec ...
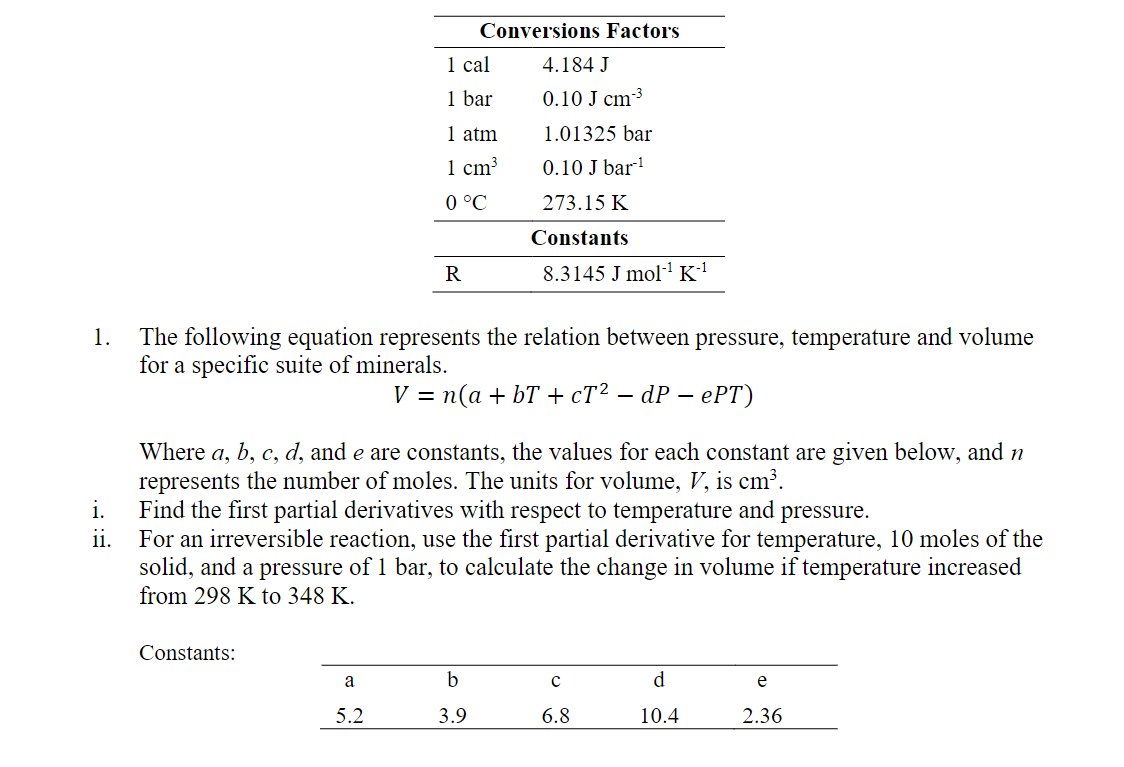
The following equation represents the relation between pressure, temperature and volume for a specific suite of minerals. Where , and are constants, the values for each constant are given below, and represents the number of moles. The units for volume, , is . Find the first partial derivatives with respect to temperature and pressure. For an irreversible reaction, use the first partial derivative for temperature, 10 moles of the solid, and a pressure of 1 bar, to calculate the change in volume if temperature increased from to . Constants: