Home /
Expert Answers /
Chemical Engineering /
the-following-elementary-gas-phase-decomposition-reaction-is-carried-out-in-a-6100l-isothermal-pf-pa195
(Solved): The following elementary gas-phase decomposition reaction is carried out in a 6100L isothermal PF ...
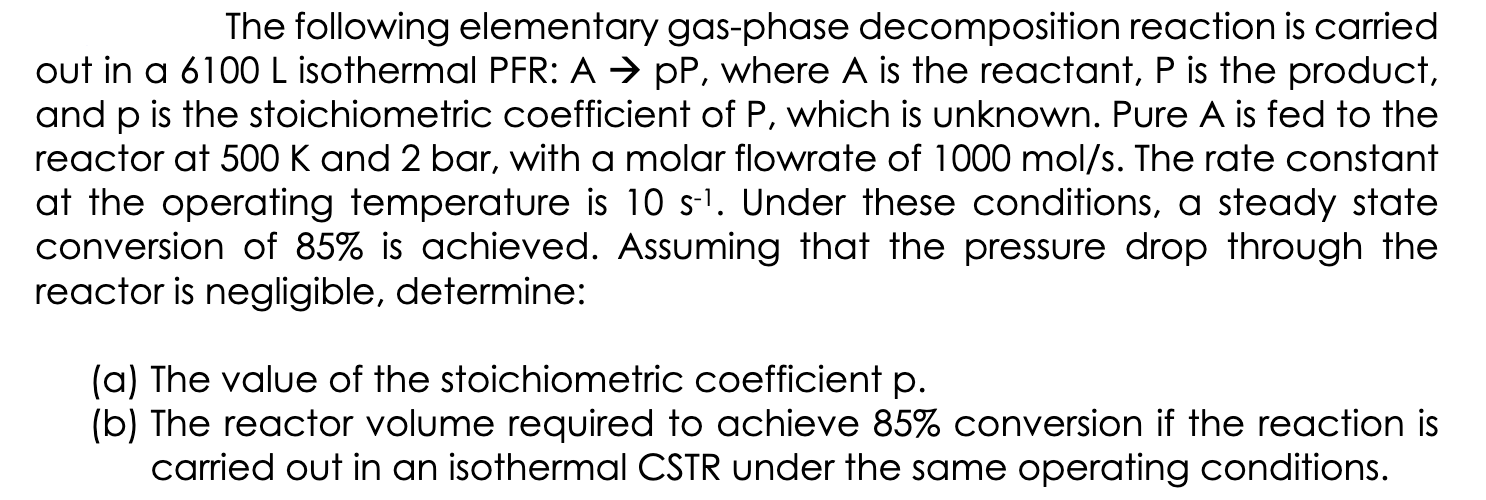
The following elementary gas-phase decomposition reaction is carried out in a isothermal PFR: , where is the reactant, is the product, and is the stoichiometric coefficient of , which is unknown. Pure is fed to the reactor at and 2 bar, with a molar flowrate of . The rate constant at the operating temperature is . Under these conditions, a steady state conversion of is achieved. Assuming that the pressure drop through the reactor is negligible, determine: (a) The value of the stoichiometric coefficient . (b) The reactor volume required to achieve conversion if the reaction is carried out in an isothermal CSTR under the same operating conditions.