Home /
Expert Answers /
Chemistry /
the-following-diagrams-represent-aqueous-solutions-of-two-monoprotic-acids-mathrm-hx-and-pa134
(Solved): The following diagrams represent aqueous solutions of two monoprotic acids, \( \mathrm{HX} \) and ...
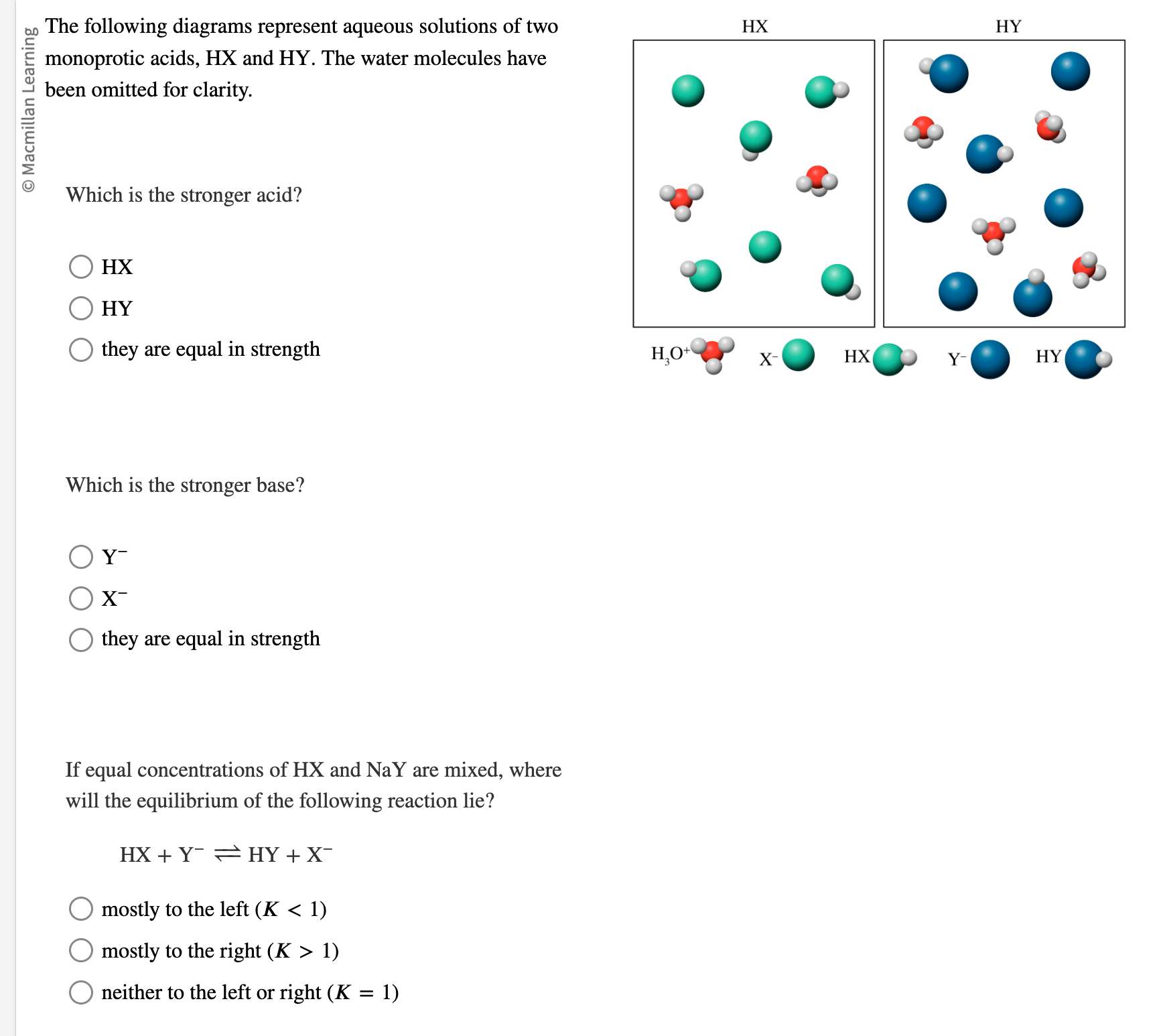
The following diagrams represent aqueous solutions of two monoprotic acids, \( \mathrm{HX} \) and \( \mathrm{HY} \). The water molecules have been omitted for clarity. Which is the stronger acid? \( \mathrm{HX} \) HY they are equal in strength Which is the stronger base? \( \mathrm{Y}^{-} \) \( \mathrm{X}^{-} \) they are equal in strength If equal concentrations of \( \mathrm{HX} \) and \( \mathrm{NaY} \) are mixed, where will the equilibrium of the following reaction lie? \[ \mathrm{HX}+\mathrm{Y}^{-} \rightleftharpoons \mathrm{HY}+\mathrm{X}^{-} \] mostly to the left ( \( K<1) \) mostly to the right \( (K>1) \) neither to the left or right \( (K=1) \)
Expert Answer
Solution to the given Question Part A to determine which acid is the stronger acid, we are required to identify the acid that has the greatest degree of dissociation. We observe that in the case of HX