Home /
Expert Answers /
Chemistry /
the-first-four-ionization-energies-ie-through-mathrm-ie-4-of-a-period-2-element-have-pa332
(Solved): The first four ionization energies (IE, through \( \mathrm{IE}_{4} \) ) of a Period 2 element have ...
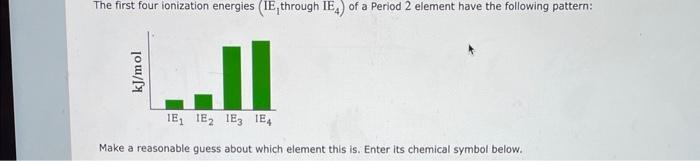
The first four ionization energies (IE, through \( \mathrm{IE}_{4} \) ) of a Period 2 element have the following pattern: Make a reasonable guess about which element this is. Enter its chemical symbol below.