Home /
Expert Answers /
Advanced Physics /
the-figure-below-shows-a-vertical-piston-cylinder-assembly-placed-on-a-hot-plate-the-piston-cylind-pa976
(Solved): The figure below shows a vertical piston-cylinder assembly placed on a hot plate. The piston-cylind ...
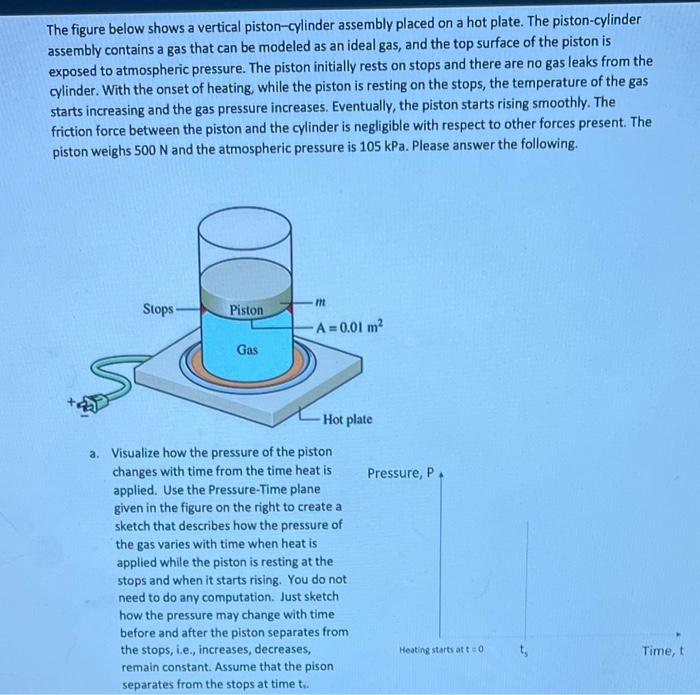
The figure below shows a vertical piston-cylinder assembly placed on a hot plate. The piston-cylinder assembly contains a gas that can be modeled as an ideal gas, and the top surface of the piston is exposed to atmospheric pressure. The piston initially rests on stops and there are no gas leaks from the cylinder. With the onset of heating, while the piston is resting on the stops, the temperature of the gas starts increasing and the gas pressure increases. Eventually, the piston starts rising smoothly. The friction force between the piston and the cylinder is negligible with respect to other forces present. The piston weighs \( 500 \mathrm{~N} \) and the atmospheric pressure is \( 105 \mathrm{kPa} \). Please answer the following. a. Visualize how the pressure of the piston changes with time from the time heat is applied. Use the Pressure-Time plane given in the figure on the right to create a sketch that describes how the pressure of the gas varies with time when heat is applied while the piston is resting at the stops and when it starts rising. You do not need to do any computation. Just sketch how the pressure may change with time before and after the piston separates from the stops, i.e., increases, decreases, remain constant. Assume that the pison separates from the stops at time \( t_{\text {. }} \)
Expert Answer
Initially at t=0 the gases are at a certain Pressure, when the heat is increased the pressure also increases as we know from ideal gas la