Home /
Expert Answers /
Chemical Engineering /
the-esterification-reaction-between-butanol-and-acetic-acid-in-the-presence-o-pa906
(Solved): The esterification reaction between butanol and acetic acid in the presence o ...
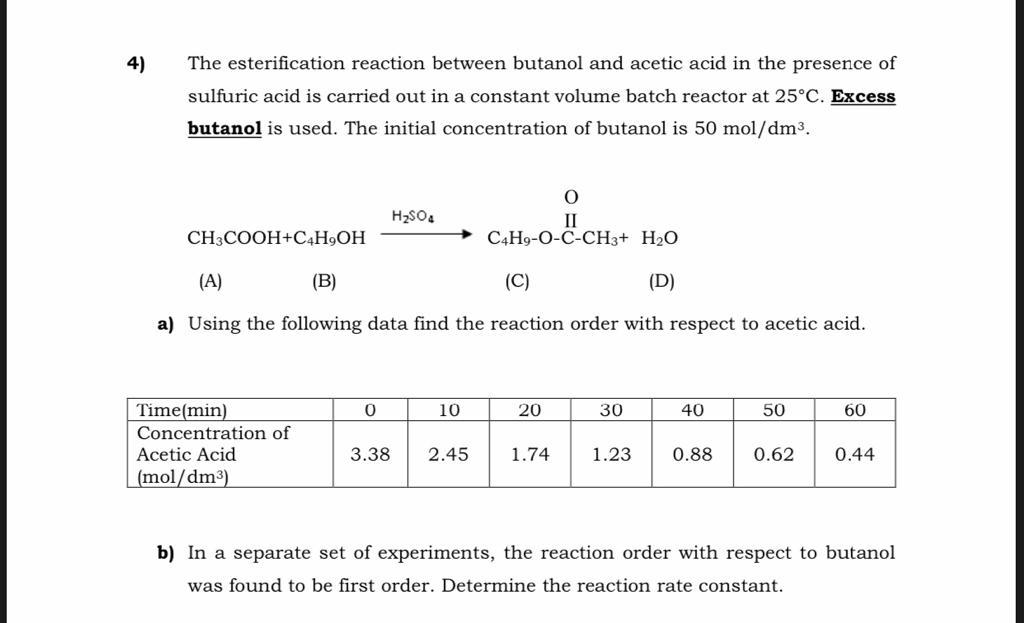
The esterification reaction between butanol and acetic acid in the presence of sulfuric acid is carried out in a constant volume batch reactor at . Excess butanol is used. The initial concentration of butanol is . a) Using the following data find the reaction order with respect to acetic acid. b) In a separate set of experiments, the reaction order with respect to butanol was found to be first order. Determine the reaction rate constant.