Home /
Expert Answers /
Chemistry /
the-equilibrium-vapour-pressure-of-a-liquid-is-dependent-on-temperature-this-relationship-between-pa331
(Solved): The equilibrium vapour pressure of a liquid is dependent on temperature. This relationship between ...
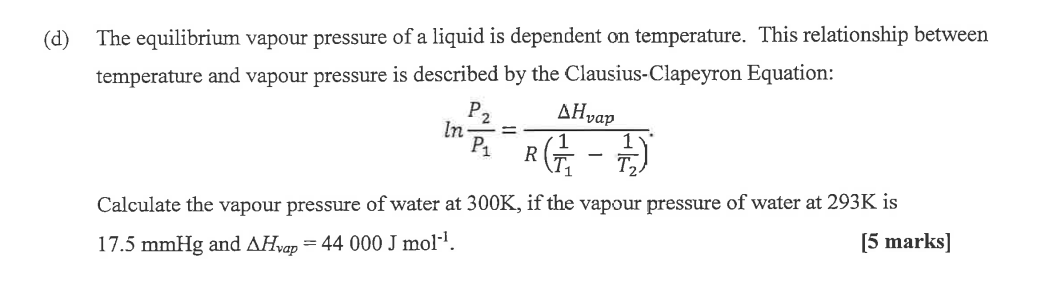
The equilibrium vapour pressure of a liquid is dependent on temperature. This relationship between temperature and vapour pressure is described by the Clausius-Clapeyron Equation: \[ \ln \frac{P_{2}}{P_{1}}=\frac{\Delta H_{\text {vap }}}{R\left(\frac{1}{T_{1}}-\frac{1}{T_{2}}\right)} . \] Calculate the vapour pressure of water at \( 300 \mathrm{~K} \), if the vapour pressure of water at \( 293 \mathrm{~K} \) is \( 17.5 \mathrm{mmHg} \) and \( \Delta H_{\text {vap }}=44000 \mathrm{~J} \mathrm{~mol}^{-1} \). [5 marks]