Home /
Expert Answers /
Chemistry /
the-equilibrium-constant-of-a-system-k-can-be-related-to-the-standard-free-energy-change-pa392
(Solved): The equilibrium constant of a system, \( K \), can be related to the standard free energy change, ...
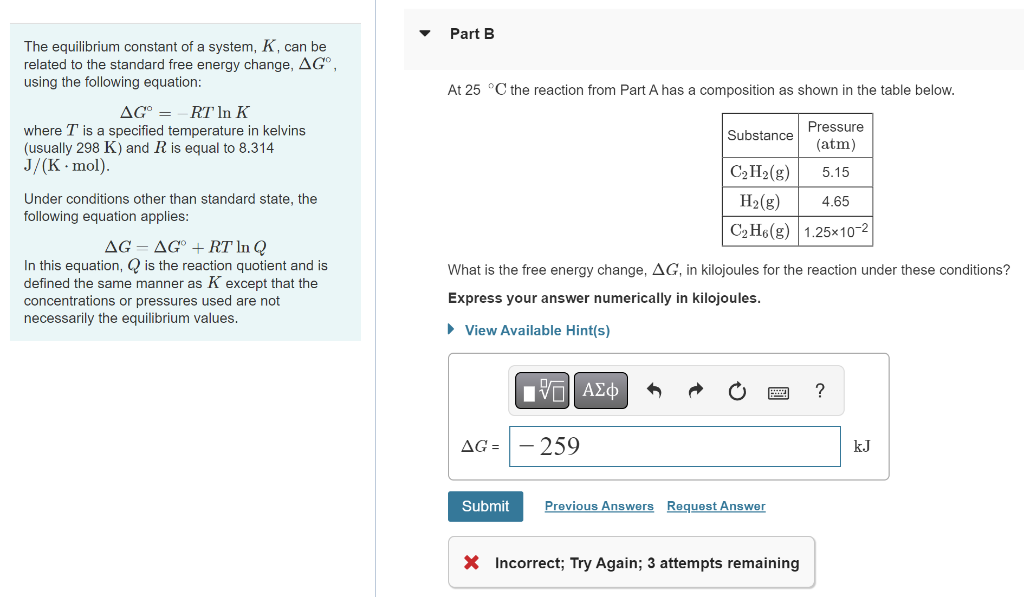
The equilibrium constant of a system, \( K \), can be related to the standard free energy change, \( \Delta G^{\circ} \), using the following equation: At \( 25^{\circ} \mathrm{C} \) the reaction from Part \( \mathrm{A} \) has a composition as shown in the table below. \[ \Delta G^{\circ}=-R T \ln K \] where \( T \) is a specified temperature in kelvins (usually \( 298 \mathrm{~K} \) ) and \( R \) is equal to \( 8.314 \) \( \mathrm{J} /(\mathrm{K} \cdot \mathrm{mol}) \). Under conditions other than standard state, the following equation applies: \[ \Delta G=\Delta G^{\circ}+R T \ln Q \] In this equation, \( Q \) is the reaction quotient and is defined the same manner as \( K \) except that the What is the free energy change, \( \Delta G \), in kilojoules for the reaction under these conditions? concentrations or pressures used are not Express your answer numerically in kilojoules. necessarily the equilibrium values. -3 Incorrect; Try Again; 3 attempts remaining