Home /
Expert Answers /
Chemistry /
the-equilibrium-constant-kc-is-calculated-using-molar-concentrations-for-gaseous-reactions-an-pa729
(Solved): The equilibrium constant, Kc, is calculated using molar concentrations. For gaseous reactions an ...
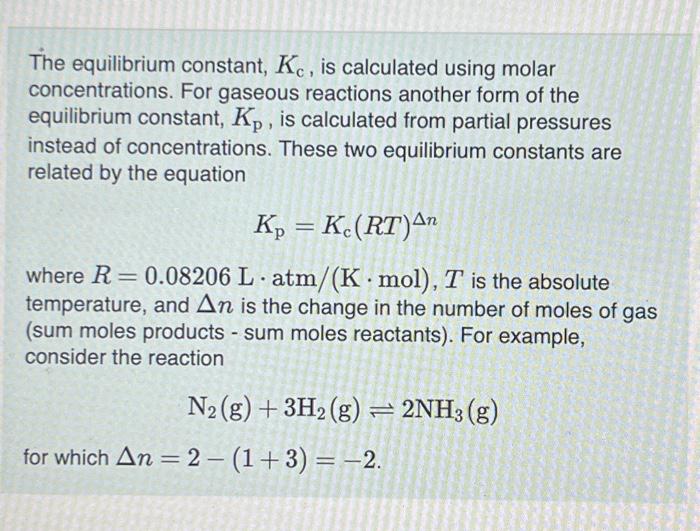

The equilibrium constant, , is calculated using molar concentrations. For gaseous reactions another form of the equilibrium constant, , is calculated from partial pressures instead of concentrations. These two equilibrium constants are related by the equation where is the absolute temperature, and is the change in the number of moles of gas (sum moles products - sum moles reactants). For example, consider the reaction for which .
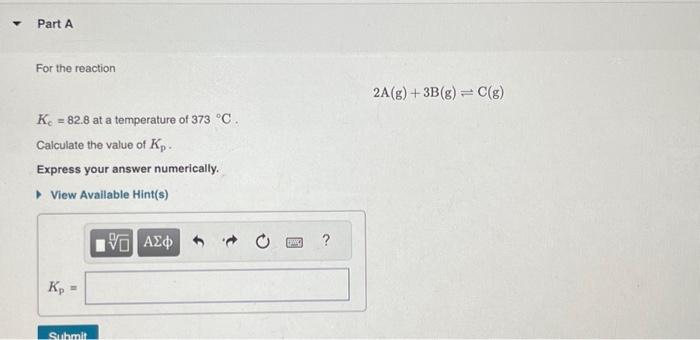
For the reaction at a temperature of . Calculate the value of . Express your answer numerically.
For the reaction at a temperature of . Calculate the value of . Express your answer numerically.