Home /
Expert Answers /
Chemistry /
the-equilibrium-constant-for-the-equation-ag-aq-2nh3-aq-ag-nh3-2-aq-is-k-2-5-pa261
(Solved): The equilibrium constant for the equation Ag+(aq)+2NH3(aq)Ag(NH3)2+(aq) is K=2.5 ...
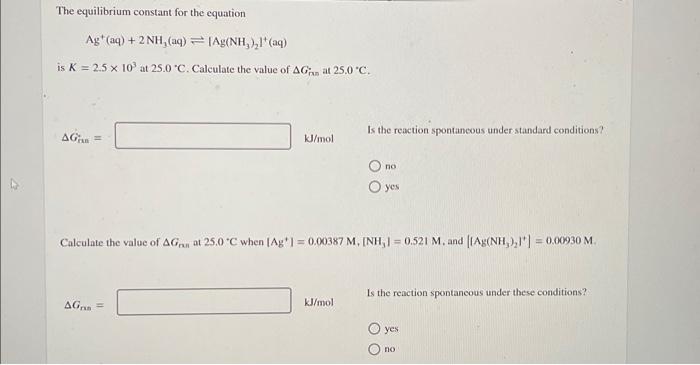
The equilibrium constant for the equation is at . Calculate the value of at . Is the reaction spontaneous under standard conditions? Calculate the value of at when , and . Is the reaction spontancous under these conditions? yes no