Home /
Expert Answers /
Chemistry /
the-enthalpy-of-vaporization-of-substance-x-is-27-0-frac-mathrm-kj-mathrm-mol-an-pa194
(Solved): The enthalpy of vaporization of Substance \( X \) is \( 27.0 \frac{\mathrm{kJ}}{\mathrm{mol}} \) an ...
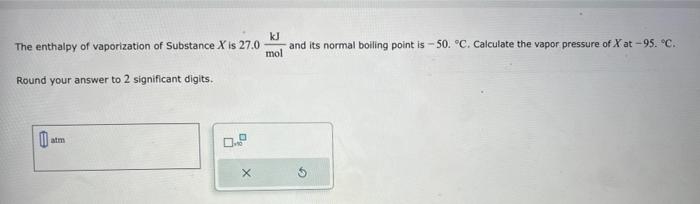
The enthalpy of vaporization of Substance \( X \) is \( 27.0 \frac{\mathrm{kJ}}{\mathrm{mol}} \) and its normal boiling point is \( -50 .^{\circ} \mathrm{C} \). Calculate the vapor pressure of \( X \) at \( -95 \). \( { }^{\circ} \mathrm{C} \). Round your answer to 2 significant digits.