Home /
Expert Answers /
Chemistry /
the-enthalpy-change-when-ammonium-nitrate-dissolves-in-water-is-called-the-heat-of-solution-and-pa572
(Solved): The enthalpy change when ammonium nitrate dissolves in water is called the heat of solution and ...
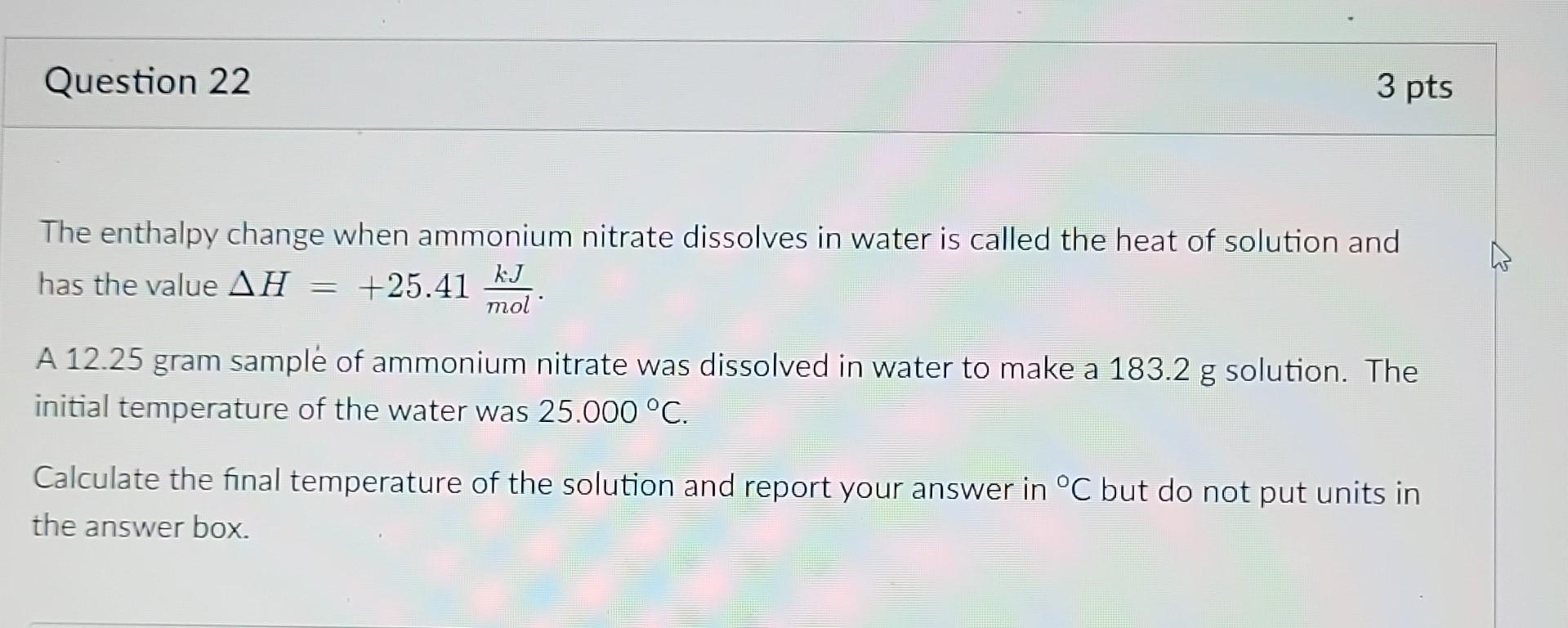
The enthalpy change when ammonium nitrate dissolves in water is called the heat of solution and has the value . A 12.25 gram sample of ammonium nitrate was dissolved in water to make a solution. The initial temperature of the water was . Calculate the final temperature of the solution and report your answer in but do not put units in the answer box.
Expert Answer
To calculate the final temperature of the solution, we can use the principle of conservation of ener...