Home /
Expert Answers /
Chemistry /
the-empirical-formula-of-a-compound-ch3f-given-that-this-same-compound-has-a-molar-mass-of-23-pa673
(Solved): The empirical formula of a compound CH3F. Given that this same compound has a molar mass of 23 ...
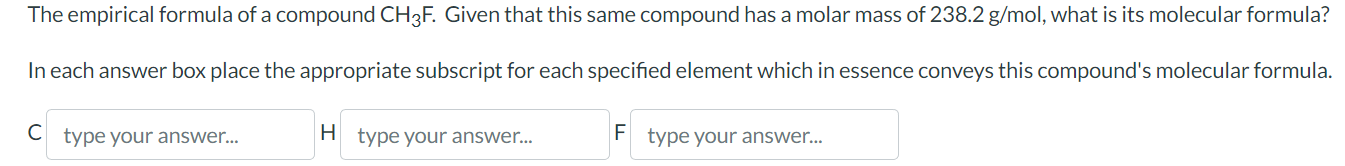
The empirical formula of a compound . Given that this same compound has a molar mass of , what is its molecular formula? In each answer box place the appropriate subscript for each specified element which in essence conveys this compound's molecular formula. C F