Home /
Expert Answers /
Chemistry /
the-dissociation-of-molecular-iodine-into-iodine-atoms-is-represented-as-12-g-21-g-at-10-pa165
(Solved): The dissociation of molecular iodine into iodine atoms is represented as 12(g)21(g) At 10 ...
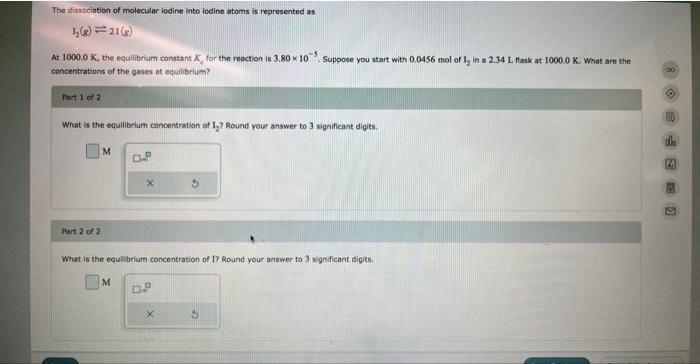
The dissociation of molecular iodine into iodine atoms is represented as At , the equilibrium constant for the reaction is . Suppose you start with of in a flark at . What are the concentrations of the gases at equilibrlum? Part 1 of 2 . What is the equilibrlum concentration of ? Round your answer to 3 significant digits. Part 2 of 2 What is the equibrium concentration of 1? Round your answer to 3 significant digits:
Expert Answer
The given reaction is The initial moles of I2 = 0.0456 mol